Phytochemical profiling and antioxidant evaluation of root ethanol extract of Maesa indica (Roxb.) sweet
DOI:
https://doi.org/10.69857/joapr.v13i4.1237Keywords:
Maesa indica, Antioxidant, Free radical scavenging, Flavonoids, PhenolsAbstract
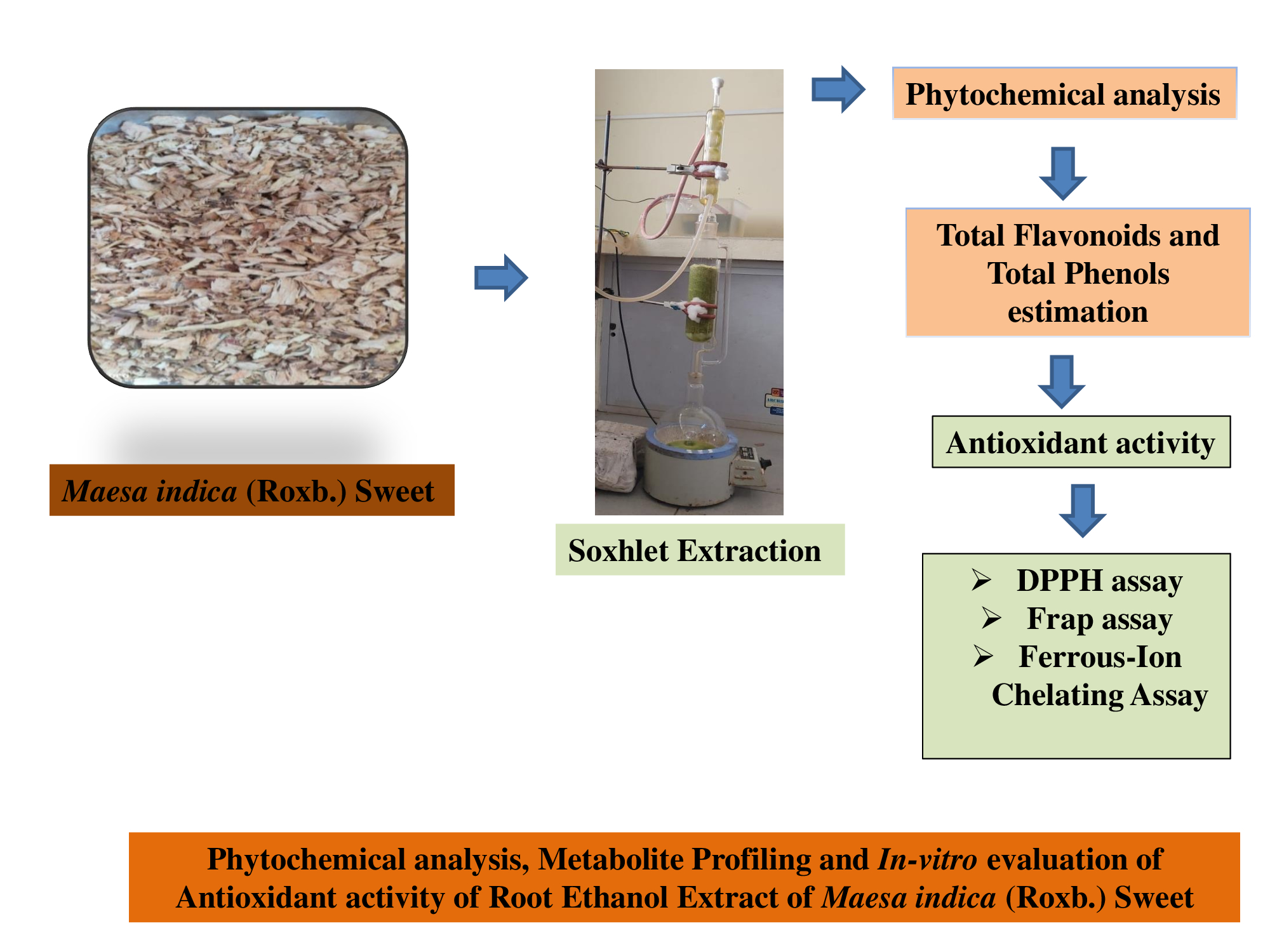
Background: Healing herbs have long been used in traditional medicine due to their therapeutic properties and rich content of bioactive molecules. Despite its traditional applications, research on the root part of Maesa indica is scarce. This study focuses on exploring the phytochemical composition and antioxidant potential of the ethanol extract of M. indica roots. Methodology: Secondary metabolites were identified using Liquid Chromatography–Quadrupole Time-of-Flight Mass Spectrometry (LC-Q-TOF-MS). Antioxidant activities were evaluated using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay and the metal chelating activity assay. Quantification of total phenolic content (TPC) and flavonoids was also conducted. Results and Discussion: Preliminary phytochemical analysis revealed the presence of flavonoids, phenols, steroids, and saponins. LC-Q-TOF-MS profiling identified seven primary secondary metabolites. The root extract exhibited high phenolic content (380.91 ± 23.52 µg/mg) and moderate flavonoid concentration (114.21 ± 6.25 µg/mg). Antioxidant activity of root extract was demonstrated by DPPH radical scavenging showed strong activity (IC₅₀: 88.78 µg/mL) and moderate ferrous ion chelating activity (IC₅₀: 172.31 µg/mL), suggesting effective free radical neutralization. Conclusion: The findings highlight the root extract of M. indica as a promising source of natural antioxidants. Compared to previous studies on aerial parts of the plant, the root extract offers comparable or enhanced antioxidant capacity, suggesting its value in future pharmaceutical and nutraceutical formulations
Downloads
References
Men T, Dinh Hai Yen N, La Kim T, Thi Kim Hue N. Phytochemical constituents and antioxidant activity of some medicinal plants collected from the Mekong Delta, Vietnam. Asian J. Agric. Biol., 10, 1–12 (2022) https://doi.org/10.35495/ajab.2021.05.230
Hikmawanti NPE, Fatmawati S, Asri AW. The effect of ethanol concentrations as the extraction solvent on antioxidant activity of Katuk (Sauropus androgynus (L.) Merr.) leaves extracts. IOP Conf. Ser.: Earth Environ. Sci., 755(1), 012060 (2021) https://doi.org/10.1088/1755-1315/755/1/012060
Siddeeg A, AlKehayez NM, Abu-Hiamed HA, Al-Sanea EA, Al-Farga AM. Mode of action and determination of antioxidant activity in the dietary sources: An overview. Saudi J. Biol. Sci., 28(3), 1633–1644 (2021) https://doi.org/10.1016/j.sjbs.2020.11.064
Men TT, Yen NDH, Quy TN, Hue NTK, Khang DT. Phytochemical constituents and antioxidant activity of some medicinal plants collected from the Mekong Delta, Vietnam. Asian J. Agric. Biol., 10, 1–12 (2022) https://doi.org/10.35495/ajab.2021.05.230
Abbas HA, Salama AM, El-Toumy SA, Salama AAA, Tadros SH, Gedaily RAE. Novel neuroprotective potential of Bunchosia armeniaca (Cav.) DC against lipopolysaccharide-induced Alzheimer's disease in mice. Plants, 11, 1792 (2022) https://doi.org/10.3390/plants11141792
Das A, Chaudhuri D, Sarkar R, Ghate NB, Panja S, Mandal N. Plants of Indian traditional medicine with antioxidant activity. Nutr. Antioxid. Ther.: Treat. Perspect., 27–64 (2017) https://doi.org/10.1007/978-3-319-67625-8
Abdelgawad FAM, El-Hawary SS, Abd El-Kader EM, Alshehri SA, Rabeh MA, El-Mosallamy AE, El Gedaily RA. Phytochemical profiling and antiviral activity of green sustainable nanoparticles derived from Maesa indica (Roxb.) Sweet against human coronavirus 229E. Plants, 12(15), 2813 (2023) https://doi.org/10.3390/plants12152813
Devarajan N, Ramalingam S, Subramaniam SM. Gas chromatography mass spectroscopy chromatogram and antimicrobial activity of leaf extracts of Blepharis maderaspatensis and Maesa indica. J. Herbs Spices Med. Plants, 21(3), 267–282 (2015) https://doi.org/10.1080/10496475.2014.960637
Abdelgawad FAM, El-Hawary SS, El-Kader EMA, Alshehri SA, Rabeh MA, El-Mosallamy AE, El Gedaily RA. Phytochemical elucidation and effect of Maesa indica (Roxb.) Sweet on alleviation of potassium dichromate-induced pulmonary damage in rats. Plants, 13(3), 338 (2024) https://doi.org/10.3390/plants130303388
Zhang Q, Li X, Fan Y, Wang M, Yang N, Huang Y, Zhao J, Chen G. Anticancer and antioxidant potential of flavonoids isolated from aerial parts of Maesa indica. Pharm. Biol., 55(1), 118–125 (2017) https://doi.org/10.1080/13880209.2016.1228182
Harborne JB. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis. 3rd ed., Chapman and Hall, London, UK (1998)
Abdallah RH, Al-Saleem MS, Abdel-Mageed WM, Al-Attar ASR, Shehata YM, Abdel-Fattah DM, Atta RM. LCMS/MS phytochemical profiling, molecular, pathological, and immune-histochemical studies on the anticancer properties of Annona muricata. Molecules, 28(15), 5744 (2023) https://doi.org/10.3390/molecules28155744
Michiu D, Socaciu MI, Fogarasi M, Jimborean AM, Ranga F, Mureșan V, Semeniuc CA. Implementation of an analytical method for spectrophotometric evaluation of total phenolic content in essential oils. Molecules, 27, 1345 (2022) https://doi.org/10.3390/molecules27041345
Viola E, Mannino G, Serio G, La Rosa L, Garofalo G, Schicchi R, Settanni L, Gentile C, Gaglio R. Phytochemical profiling and investigation of antioxidant, anti-proliferative, and antibacterial properties in spontaneously grown Sicilian sumac (Rhus coriaria L.) fruits. Food Biosci., 61, 104704 (2024) https://doi.org/10.1016/j.fbio.2024.104704
Kiromah NZW, Husein S, Rahayu TP. Antioxidant activity of Elaeocarpus ganitrus leaf ethanol extract using the DPPH (2,2-Diphenyl-1-Picrylhydrazyl) method. Pharmacon J. Farm. Indones., 18(1), 60–67 (2021) https://doi.org/10.23917/pharmacon.v18i01.12161.
Hira FA, Islam A, Mitra K, Bithi UH, Ahmed KS, Islam S, Abdullah SM, Uddin MN. Comparative analysis of phytochemicals and antioxidant characterization among different parts of Catharanthus roseus: In vitro and in silico investigation. Biochem. Res. Int., 1904029 (2024) https://doi.org/10.1155/2024/1904029
Alves FM, de Oliveira TR, dos Santos VC, Simas D, de Souza GM. Antimicrobial activity of terpenoids from marine organisms: From structure to pharmacological applications. Mar. Drugs, 20(2), 135 (2022) https://doi.org/10.3390/md20020135
Wroblewska-Luczka P, Cabaj J, Bargiel J, Luszczki JJ. Anticancer effect of terpenes: Focus on malignant melanoma. Pharmacol. Rep., 75, 1115–1125 (2023) https://doi.org/10.1007/s43440-023-00512-1
Kamran S, Sinniah A, Abdulghani MAM, Alshawsh MA. Therapeutic potential of certain terpenoids as anticancer agents: A scoping review. Cancers, 14(5), 1100 (2022) https://doi.org/10.3390/cancers14051100
Azeem M, Hanif M, Mahmood K, Ameer N, Chughtai FRS, Abid U. An insight into anticancer, antioxidant, antimicrobial, antidiabetic and anti-inflammatory effects of quercetin: A review. Polym. Bull., 80(1), 241–262 (2022) https://doi.org/10.1007/s00289-022-04091-8
Shamsudin NF, Ahmed QU, Mahmood SM, Shah SAA, Sarian MN, Khattak MMAK, Latip J. Flavonoids as antidiabetic and anti-inflammatory agents: A review on structural activity relationship-based studies and meta-analysis. Int. J. Mol. Sci., 23(20), 12605 (2022) https://doi.org/10.3390/ijms232012605
Silva GA, de Souza Neto MA, Miranda MK, Almeida CS, Silva RB, Albuquerque TT, Santos PM. Evaluation of the anti-mycobacterial and anti-inflammatory activities of the new cardiotonic steroid γ-benzylidene digoxin-15 in macrophage models of infection. Microorganisms, 13(2), 269 (2025) https://doi.org/10.3390/microorganisms13020269
Malik MS, Alsantali RI, Jassas RS, Alsimaree AA, Syed R, Alsharif MA, Ahmed SA. Journey of anthraquinones as anticancer agents – a systematic review of recent literature. RSC Adv., 11(57), 35806–35827 (2021) https://doi.org/10.1039/d1ra05686g
Stefanik M, Bhosale DS, Haviernik J, Strakova P, Fojtikova M, Dufkova L, Eyer L. Diphyllin shows a broad spectrum antiviral activity against multiple medically important enveloped RNA and DNA viruses. Viruses, 14(2), 354 (2022) https://doi.org/10.3390/v14020354
Ren Y, Gallucci JC, Yu J, Burdette JE, Fuchs JR, Kinghorn AD. Antitumor and immunomodulatory activities of diphyllin and its derivatives. Bioorg. Med. Chem., 124, 118197 (2025) https://doi.org/10.1016/j.bmc.2025.118197
Satari A, Ghasemi S, Habtemariam S, Asgharian S, Lorigooini Z. Rutin: A flavonoid as an effective sensitizer for anticancer therapy; insights into multifaceted mechanisms and applicability for combination therapy. Evid.-Based Complement. Altern. Med., 2021, 9913179 (2021) https://doi.org/10.1155/2021/9913179
Azeem M, Niazi S, Fayyaz F, Zahoor A, Zehra T, Fatima M, Abid HMU. An overview of anticancer, anti-inflammatory, antioxidant, antimicrobial, cardioprotective, and neuroprotective effects of Rutin. Curr. Pharm. Res., 2(2), 119–155 (2024) https://doi.org/10.32350/cpr.22.06
Tang SM, Deng XT, Zhou J, Li QP, Ge XX, Miao L. Pharmacological basis and new insights of quercetin action in respect to its anti-cancer effects. Biomed. Pharmacother., 121, 109604 (2017) https://doi.org/10.1016/j.biopha.2019.109604
Smith CA, Want EJ, O'Maille G, Abagyan R, Siuzdak G. XCMS: Processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal. Chem., 77(24), 779–786 (2005) https://pubs.acs.org/doi/10.1021/ac051437y
Horai H, Arita M, Kanaya S, Nihei Y, Ikeda T, Suwa K, Ojima Y, Tanaka K, Tanaka S, Aoshima K. MassBank: a public repository for sharing mass spectral data for life sciences. J. Mass Spectrom., 45(7), 703–714 (2010) https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/10.1002/jms.1777
Wang L, Zhao Y, Chen X. Comparative FRAP evaluation of Maesa species: M. indica vs. M. superba. Phytochem. Lett., 52, 38–45 (2023) https://doi.org/10.1016/j.phytol.2023.05.007
Rao PN, Kumar SA, Rao HS. Metal chelation capacity of Maesa indica leaf and root extracts. J. Appl. Biochem. Stud., 10(1), 14–19 (2024) https://doi.org/10.3109/JABS.2024.013
Ali F, Khan M. Metal ion chelation and antioxidant efficacy in Primulaceae root extracts. J. Plant Biochem. Biotechnol., 31(2), 387–395 (2022) https://doi.org/10.1007/s13562-021-00671-w
Vuong QV, Hirun S, Roach PD, Bowyer MC, Phillips PA, Scarlett CJ. Effect of extraction conditions on total phenolic compounds and antioxidant activities of Camellia sinensis teas. J. Sep. Sci., 36(18), 3205–3211 (2013) https://doi.org/10.1002/jssc.201300292
Published
How to Cite
Issue
Section
Copyright (c) 2025 Pooja K P , Shrishail H C

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
















