Neuroprotective potential of methanolic leaf extracts of Celosia cristata and Callistemon citrinus on scopolamine-induced amnesia in swiss albino mice
DOI:
https://doi.org/10.69857/joapr.v13i4.1139Keywords:
Acetylcholinesterase, Amnesia, Memory, Morris water maze, NeuroprotectiveAbstract
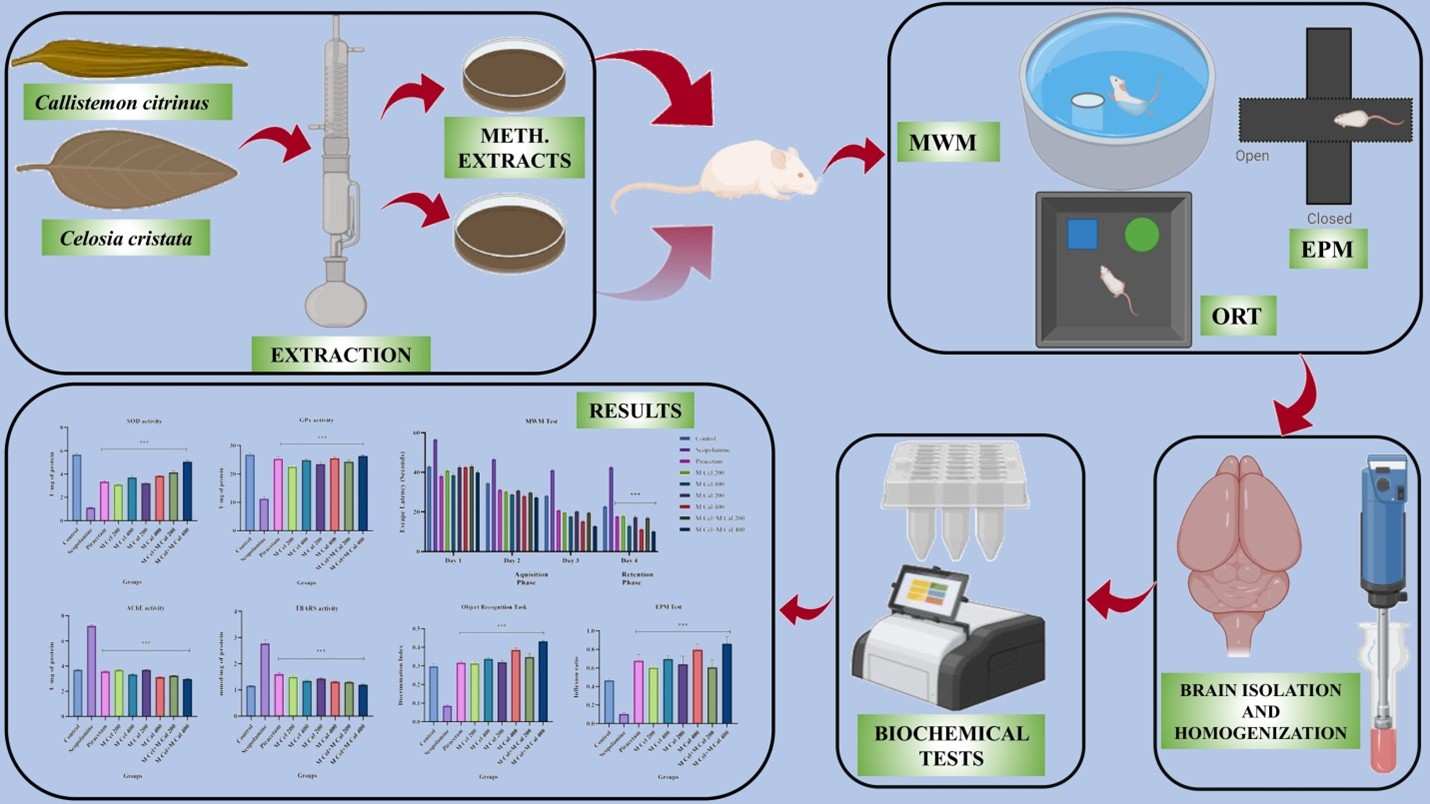
Background: The primary reason for memory loss is Alzheimer’s disease, a progressive neurodegenerative condition in specific brain parts. This study aims to illustrate the relative enhancement of memory, along with the neuroprotective and antioxidant properties of methanolic leaf extracts from Celosia cristata and Callistemon citrinus in scopolamine-induced amnesia in mice. Methodology: Methanolic extracts of the leaves of Celosia cristata and Callistemon citrinus were evaluated for their effects on scopolamine-induced impaired learning and memory in Swiss albino mice using behavioral animal models, including the Morris water maze (MWM), elevated plus maze (EPM), and object recognition task (ORT). Antioxidants such as Superoxide dismutase (SOD), Glutathione peroxidase (GPx), Thiobarbituric acid reactive substance (TBARS), and acetylcholinesterase (AChE) were also assessed at different doses, i.e., 200 and 400 mg/Kg of methanolic extracts of Celosia cristata and Callistemon citrinus, as well as their combinations. Results and Discussion: The various doses of Celosia cristata and Callistemon citrinus methanolic leaf extracts significantly modified scopolamine effects in experimental animals. Extracts significantly decreased escape latency (ELT) in the MWM test. Inflexion ratio (IR) in the EPM test was significantly raised by extracts, as well as the discrimination index (DI) in ORT. The SOD and GPx levels were significantly enhanced whereas TBARS significantly reduced by extracts. The significant reduced level of AChE was reported in extract treated mice. The extracts from both plants exhibited significant results at different doses (200 mg/kg and 400 mg/kg) and combination of both plant extracts (MCel+MCal 400) at 400mg/kg dose showed most significant result. Conclusion: The results revealed that methanolic leaf extracts of Celosia cristata and Callistemon citrinus hold potent antiamnesic effects.
Downloads
References
Parle M, Vasudevan M. Memory Enhancing Activity of Abana: An Indian Ayurvedic Poly-Herbal Formulation. Journal of Health Science, 53(1), 43–52 (2007) https://doi.org/10.1248/jhs.53.43
Dhingra D, Parle M, Kulkarni SK. Memory enhancing activity of Glycyrrhiza glabra in mice. Journal of Ethnopharmacology, 91(2–3), 361–365 (2004) https://doi.org/10.1016/j.jep.2004.01.016
Nabeshima T. Chapter 48: Behavioral aspects of cholinergic transmission: role of basal forebrain cholinergic system in learning and memory, 405–411 (1993) https://doi.org/10.1016/S0079-6123(08)62424-3
Higashida A, Ogawa N. Differences in the acquisition process and the effect of scopolamine on radial maze performance in three strains of rats. Pharmacology Biochemistry and Behavior, 27(3), 483–489 (1987) https://doi.org/10.1016/0091-3057(87)90352-2
Shaji KS, Arun Kishore NR, Lal KP, Prince M. Revealing a hidden problem. An evaluation of a community dementia case‐finding program from the Indian 10/66 dementia research network. International Journal of Geriatric Psychiatry, 17(3), 222–225 (2002) https://doi.org/10.1002/gps.553
Kawas C, Gray S, Brookmeyer R, Fozard J, Zonderman A. Age-specific incidence rates of Alzheimer’s disease. Neurology, 54(11), 2072–2077 (2000) https://doi.org/10.1212/WNL.54.11.2072
Vas CJ, Pinto C, Panikker D, Noronha S, Deshpande N, Kulkarni L, Sachdeva S. Prevalence of Dementia in an Urban Indian Population. International Psychogeriatrics, 13(4), 439–450 (2001) https://doi.org/10.1017/S1041610201007852
Ngai DN, Kibiti CM, Ngugi MP. Spatial memory-enhancing effects and antioxidant activities of leaf and stem bark methanol extracts of Prunus africana in scopolamine-treated mice. Journal of Herbmed Pharmacology, 12(2), 315–326 (2023) https://doi.org/10.34172/jhp.2023.33
Vyas S, Kothari SL, Kachhwaha S. Nootropic medicinal plants: Therapeutic alternatives for Alzheimer’s disease. Journal of Herbal Medicine, 17–18, 100291 (2019). https://doi.org/10.1016/j.hermed.2019.100291
Mukherjee PK, Kumar V, Mal M, Houghton PJ. Acetylcholinesterase inhibitors from plants. Phytomedicine, 14(4), 289–300 (2007) https://doi.org/10.1016/j.phymed.2007.02.002
Uddin MdS et al. Exploring the multimodal role of phytochemicals in the modulation of cellular signaling pathways to combat age-related neurodegeneration. Science of The Total Environment, 725, 138313 (2020) https://doi.org/10.1016/j.scitotenv.2020.138313
Goyal PK, Jain R, Jain S, Sharma A. A Review on biological and phytochemical investigation of plant genus Callistimon. Asian Pacific Journal of Tropical Biomedicine, 2(3), S1906–S1909 (2012) https://doi.org/10.1016/S2221-1691(12)60519-X
Chauhan BS, Tiwari A, Bhadauria A. A study on phytochemical extraction of Aloe vera. International Journal Of Agricultural Sciences, 18(2), 786–792 (2022) https://doi.org/10.15740/HAS/IJAS/18.2/786-792
Adam OAO, Abadi RSM, Ayoub SMH. The Effect of Extraction method and Solvents on yield and Antioxidant Activity of Certain Sudanese Medicinal Plant Extracts. The Journal of Phytopharmacology, 8(5), 248–252 (2019) https://doi.org/10.31254/phyto.2019.8507
Harborne J.B. (1998). Textbook of Phytochemical Methods. A Guide to Modern Techniques of Plant Analysis (5th ed.). Chapman and Hall Ltd, London.
William Charles Evans. (2009). Trease and Evans’ Pharmacognosy. Elsvier.
Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. Journal of Neuroscience Methods, 11(1), 47–60 (1984) https://doi.org/10.1016/0165-0270(84)90007-4
Vogel HG. (2002). Drug discovery and evaluation: pharmacological assays. Springer.
Pahaye DB et al. Neuroprotective and Antiamnesic Effects of Mitragyna inermis Willd (Rubiaceae) on Scopolamine-Induced Memory Impairment in Mice. Behavioural Neurology, 1–11 (2017) https://doi.org/10.1155/2017/5952897
Prabhakar S, Saraf MK, Pandhi P, Anand A. Bacopa monniera exerts antiamnesic effect on diazepam-induced anterograde amnesia in mice. Psychopharmacology, 200(1), 27–37 (2008) https://doi.org/10.1007/s00213-007-1049-8
Budzynska B, Boguszewska-Czubara A, Kruk-Slomka M, Skalicka-Wozniak K, Michalak A, Musik I, Biala G. Effects of imperatorin on scopolamine-induced cognitive impairment and oxidative stress in mice. Psychopharmacology, 232(5), 931–942 (2015) https://doi.org/10.1007/s00213-014-3728-6
Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry, 95(2), 351–358 (1979) https://doi.org/10.1016/0003-2697(79)90738-3
Ellman GL, Courtney KD, Andres V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemical Pharmacology, 7(2), 88–95 (1961) https://doi.org/10.1016/0006-2952(61)90145-9
Pohanka M, Hrabinova M, Kuca K, Simonato JP. Assessment of Acetylcholinesterase Activity Using Indoxylacetate and Comparison with the Standard Ellman’s Method. International Journal of Molecular Sciences, 12(4), 2631–2640 (2011) https://doi.org/10.3390/ijms12042631
Han R et al. Reversal of scopolamine-induced spatial and recognition memory deficits in mice by novel multifunctional dimers bis-cognitins. Brain Research, 1470, 59–68 (2012) https://doi.org/10.1016/j.brainres.2012.06.015
Rabiei Z, Mokhtari S, Asgharzade S, Gholami M, Rahnama S, Rafieian-kopaei M. Inhibitory effect of Thymus vulgaris extract on memory impairment induced by scopolamine in rat. Asian Pacific Journal of Tropical Biomedicine, 5(10), 845–851 (2015) https://doi.org/10.1016/j.apjtb.2015.07.006
D’Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Research Reviews, 36(1), 60–90 (2001) https://doi.org/10.1016/S0165-0173(01)00067-4
Beatty WW, Butters N, Janowsky DS. Patterns of memory failure after scopolamine treatment: Implications for cholinergic hypotheses of dementia. Behavioral and Neural Biology, 45(2), 196–211 (1986) https://doi.org/10.1016/S0163-1047(86)90772-7
Collerton D. Cholinergic function and intellectual decline in Alzheimer’s disease. Neuroscience, 19(1), 1–28 (1986) https://doi.org/10.1016/0306-4522(86)90002-3
Kulkarni K, Kasture S, Mengi S. Efficacy study of Prunus amygdalus (almond) nuts in scopolamine-induced amnesia in rats. Indian Journal of Pharmacology, 42(3), 168 (2010) https://doi.org/10.4103/0253-7613.66841
Debruin N, Pouzet B. Beneficial effects of galantamine on performance in the object recognition task in Swiss mice: Deficits induced by scopolamine and by prolonging the retention interval. Pharmacology Biochemistry and Behavior, 85(1), 253–260 (2006) https://doi.org/10.1016/j.pbb.2006.08.007
Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: a new perspective. Nature Reviews Neuroscience, 8(11), 872–883 (2007) https://doi.org/10.1038/nrn2154
Butterfield DA, Drake J, Pocernich C, Castegna A. Evidence of oxidative damage in Alzheimer’s disease brain: central role for amyloid β-peptide. Trends in Molecular Medicine, 7(12), 548–554 (2001) https://doi.org/10.1016/S1471-4914(01)02173-6
Kwon SH, Ma SX, Joo HJ, Lee SY, Jang CG. Inhibitory Effects of Eucommia ulmoides Oliv. Bark on Scopolamine-Induced Learning and Memory Deficits in Mice. Biomolecules and Therapeutics, 21(6), 462–469 (2013) https://doi.org/10.4062/biomolther.2013.074
Ben-Barak J, Dudai Y. Scopolamine induces an increase in muscarinic receptor level in rat hippocampus. Brain Research, 193(1), 309–313 (1980) https://doi.org/10.1016/0006-8993(80)90973-7
Jeong EJ, Lee KY, Kim SH, Sung SH, Kim YC. Cognitive-enhancing and antioxidant activities of iridoid glycosides from Scrophularia buergeriana in scopolamine-treated mice. European Journal of Pharmacology, 588(1), 78–84 (2008) https://doi.org/10.1016/j.ejphar.2008.04.015
Saliu JK, Bawa-Allah KA. Toxicological Effects of Lead and Zinc on the Antioxidant Enzyme Activities of Post Juvenile Clarias gariepinus. Resources and Environment, 2(1), 21–26 (2012) https://doi.org/10.5923/j.re.20120201.03
Al-Enazi MM. Combined Therapy of Rutin and Silymarin has More Protective Effects on Streptozotocin-Induced Oxidative Stress in Rats. Journal of Applied Pharmaceutical Science, 4(01), 021-028 (2014) https://doi.org/10.7324/JAPS.2014.40104
Barja de Quiroga G, Pérez-Campo R, López Torres M. Anti-oxidant defences and peroxidation in liver and brain of aged rats. Biochemical Journal, 272(1), 247–250 (1990) https://doi.org/10.1042/bj2720247
Lee KH, Cha M, Lee BH. Neuroprotective Effect of Antioxidants in the Brain. International Journal of Molecular Sciences, 21(19), 7152 (2020) https://doi.org/10.3390/ijms21197152
Singh M, Kaur M, Kukreja H, Chugh R, Silakari O, Singh, D. Acetylcholinesterase inhibitors as Alzheimer therapy: From nerve toxins to neuroprotection. European Journal of Medicinal Chemistry, 70, 165–188 (2013) https://doi.org/10.1016/j.ejmech.2013.09.050
Goverdhan P, Sravanthi A, Mamatha T. Neuroprotective Effects of Meloxicam and Selegiline in Scopolamine-Induced Cognitive Impairment and Oxidative Stress. International Journal of Alzheimer’s Disease, 2012, 1–8 (2012) https://doi.org/10.1155/2012/974013
De Lima EP et al. Polyphenols, Alkaloids, and Terpenoids Against Neurodegeneration: Evaluating the Neuroprotective Effects of Phytocompounds Through a Comprehensive Review of the Current Evidence. Metabolites, 15(2), 124 (2025) https://doi.org/10.3390/metabo15020124
Spagnuolo C, Napolitano M, Tedesco I, Moccia S, Milito A, Luigi Russo G. Neuroprotective Role of Natural Polyphenols. Current Topics in Medicinal Chemistry, 16(17), 1943–1950 (2016) https://doi.org/10.2174/1568026616666160204122449
Dajas F et al. Neuroprotection by flavonoids. Brazilian Journal of Medical and Biological Research, 36(12), 1613–1620 (2003) https://doi.org/10.1590/S0100-879X2003001200002
Kabir MdT et al. Exploring the Anti-Neuroinflammatory Potential of Steroid and Terpenoid-Derived Phytochemicals to Combat Alzheimer’s Disease. Current Pharmaceutical Design, 27(22), 2635–2647 (2021) https://doi.org/10.2174/1381612826666210101152352
Published
How to Cite
Issue
Section
Copyright (c) 2025 Vishwambhar Mishra, Bhupendra Chauhan, Sanjiv Kumar Chaudhri, Deepika Rani

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
















