Formulation, optimization, and standardization of orodispersible herbal tablets using design of experiments (DOE)
DOI:
https://doi.org/10.69857/joapr.v13i4.973Keywords:
Orodispersible, Sodium starch glycolate, microcrystalline cellulose (MCC), Crospovidone, Design of experimentAbstract
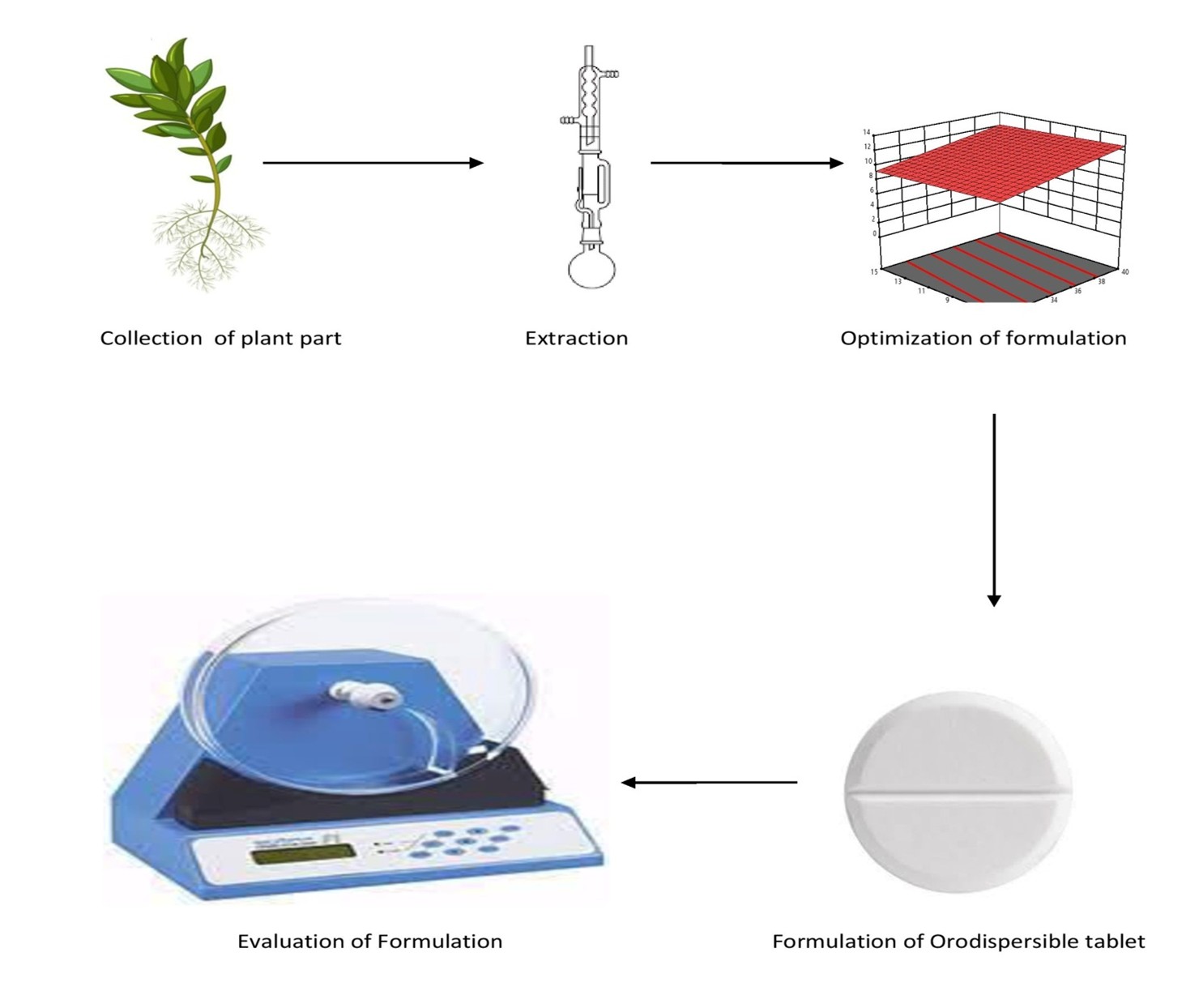
Background: This study aimed to develop and optimize an orodispersible herbal tablet incorporating Achyranthes aspera Linn extract. Sodium Starch Glycolate and Crospovidone were employed as superdisintegrants to promote rapid tablet disintegration, while β-cyclodextrin was utilized to enhance the solubility of specific constituents within the extract. The optimized formulation exhibited a rapid disintegration time of 1.805 seconds and achieved a cumulative drug release of 98.04%, indicating improved dissolution and potential enhancement in oral bioavailability. Methodology: Orodispersible tablets were formulated using Design of Experiments (DOE) software, with crospovidone and sodium starch glycolate as independent variables, and time of disintegration and cumulative drug release as dependent variables. The formulation was evaluated for weight variation, uniformity, hardness, wetting time, and in vitro dispersion time. Result & Discussion: The optimized F6 batch of orodispersible herbal tablets demonstrated the following characteristics: hardness of 2.98 kg/cm², friability of 0.58%, weight variation of 3.319%, disintegration time of 13.805 seconds, wetting time of 34.4 seconds, content uniformity of 99.5%, water absorption of 36%, and cumulative drug release of 98.04%, all within the permissible limits as per official pharmacopoeialstandards. Conclusion: The study concludes that crospovidone and sodium starch glycolate effectively reduce disintegration time and improve cumulative drug release. These findings validate the reliability of the model, with minor deviations attributed to experimental variability.
Downloads
References
Ghourichay MP, Kiaie SH, Nokhodchi A, Javadzadeh Y. Formulation and Quality Control of Orally Disintegrating Tablets (ODTs), Recent Advances and Perspectives. Biomed Res Int,7(1), 51-58 (2021) https://doi.org/10.1155/2021/6618934.
Chinwala M. Recent Formulation Advances and Therapeutic Usefulness of Orally Disintegrating Tablets (ODTs). Pharmacy (Basel), 8(4), 186 (2020) https://doi.org/10.3390/pharmacy8040186.
Kumar D, Kumar A, Malik J, Semwal P. Formulation and Evaluation of Fast Dissolving Tablets Uncoated Tablets of Drotaverine HCL. World Journal of Pharmaceutical Research, 9(1), 1716-1727 (2020) https://doi.org/10.20959/wjpps20201-16560
Kharade SB, Bais SK. Review on Quality Aspects of Herbal Drugs and its Formulations. Internationl journal of Advanced Research in Science Communication and Technology, 3(4), 161-175 (2023) https://doi.org/10.18231/j.joapr.2024.12.2.124.130.
Sharma S, Shikha B. A review: An overview of Natural superdisintegrants Research. Journal of Topical and Cosmetic Sciences, 12(1), 13-24 (2021) https://doi.org/10.52711/2321-5844.2021.00003.
Mahale S, Tayde M.Design and evaluation of cost-effective oro-dispersible tablets of venlafaxine hydrochloride by effervescent method. Journal of Applied Pharmaceutical Research, 12(3), 46-55 (2024) https://doi.org/10.69857/joapr.v12i3.501.
Gosavi OR. Exploring the therapeutic potential of Achyranthes aspera: A comprehensive review. WJBPHS,18(2), 239-245 (2024) https://doi.org/10.30574/wjbphs.2024.18.2.0230.
Paula A, Todoran N. Development and Evaluation of Cannabidiol Orodispersible Tablets Using a 23-Factorial Design. Pharmaceutics, 14(7), 1467 (2022) https://doi.org/10.3390/pharmaceutics14071467
Kaushik A. Protective effect of Achyranthes aspera against compound 48/80, histamine and ovalbumin-induced allergic disorders in murine model. Molecular Biology Reports, 51(1), 202 (2024) https://doi.org/10.1007/s11033-023-09137-2.
Kumar A, Shukla AK. Evaluation of anti-asthmatic activity of Achyranthes aspera in ovalbumin-induced murine model,Molecular Biology Reports, 51(1), 89–98(2024) https://doi.org/10.1007/s11033-023-09137-2
Sharma R. Modulatory effects of Achyranthes aspera extract on Th2 cytokines in allergic airway inflammation: An experimental study, J Ethnopharmacol, 305, 116058 (2023) https://doi.org/10.1016/j.jep.2022.116058.
Patel Patel M, Sutar N, Goyal R. Phytochemical screening and bronchodilatory potential of Achyranthes aspera: Implications for asthma treatment, Pharmacogn Rev, 6(32), 99–106.
(2022) https://doi.org/10.4103/phrev.phrev_32_21
Shinde G, Kunkulol R. Evaluation of Anti-asthmatic activity of Achyranthes aspera Linn root Extract. Research Journal of Pharmacy and Technology, 16(5), 2287-0 (2023) https://doi.org/10.52711/0974-360X.2023.00375.
Bhat LR, Vora AT, Damle MC. Simultaneous estimation of nebivolol hydrochloride and amlodipine besylate in bulk and combined tablet dosage form by ultraviolet spectrophotometry. Indian Drugs, 44(12), 915 919 (2007) https://doi.org/10.53879/id.62.01.149965
Kumar D, Kumar A, Malik J, Semwal P. Formulation and Evaluation of Fast Dissolving Uncoated Tablets of Drotaverine HCL. World Journal of Pharmaceutical Research, 9(1), 1716-1727 (2020) https://doi.org/10.20959/wjpps20201-16560
Jadhav N, Jadhav R, Jadhav S, Jadhav R. Formulation and Evaluation of Herbal Tablets Containing Extract of Trigonella foenum-graecum Linn. International Journal of Pharmaceutical Sciences and Research, 12(7), 3880-3888 (2021). https://doi.org/10.13040/IJPSR.0975-8232.12(7).3880-88
Chaudhari S, Bhalerao K, Nandgude T. Formulation and Evaluation of Herbal Tablets Containing Ethanolic Extract of Terminalia chebula Retz. Journal of Drug Delivery and Therapeutics, 10(6-s), 190-195 (2020). https://doi.org/10.22270/jddt.v10i6-s.4360
Patel R, Patel M, Patel H, Suthar M. Preformulation Studies and Formulation Development of Herbal Tablet Containing Aegle marmelos. International Journal of Pharmaceutical Sciences Review and Research, 64(1), 24-30 (2020) https://doi.org/10.47583/ijpsrr.2020.v64i01.005
Patil DJ, Jahagirdar SN, Mandhare SB. Validated Stability Indicating RP-HPLC Method for the Quantification of Process Related Impurities of Solifenacin and Mirabegron in Pharmaceutical Formulations. International Journal of Drug Delivery Technology,14(1), 55-60 (2024) https://doi.org/10.25258/ijddt.14.1.10
Dawadi S, Pandey B, Nepal S. Formulation and evaluation of orally disintegrating tablet of metoclopramide hydrochloride. World J Curr Med Pharm Res, 2(6), 322–329 (2020) https://doi.org/10.37022/wjcmpr.vi.162.
Vanhere KA. Comprehensive Review of Effervescent Tablets. Journal of Drug Delivery and Therapeutics, 13(7), 141 – 50 (2023) https://doi.org/10.22270/jddt.v13i7.6120.
Channi NM, Hurkadale PJ, Bolmal UB, Bidikar CM.Formulation Design and Optimization of Poly-Herbal Chewable Tablets Using JMP Tool. Pharmacognosy, 2(1), 3 (2023) https://doi.org/10.35702/Pharmacogn.10003.
Napit M. Formulation and Evaluation of Herbal Tablets Containing Ethanolic Extract of Acalypha indica – A Quality by Design (QbD) Approach. Research Gate Preprint, 1(1), 1-10 (2024) https://doi.org/10.13140/RG.2.2.15967.82082.
Gupta D, Biswas AA, Sahu RC, Arora S, Agrawal AK. Advancing Pharmaceutical Intelligence via Computational Prognostication of In-Vitro Parameters of Fast Disintegrating Tablets Using Machine Learning Models. Eur J Pharm Biopharm, 198(1), 112-124 (2024) https://doi.org/10.1016/j.ejpb.2024.09.005.
Rather GJ, Hamiduddin, Ikram M, Naquibuddin M. Formulation and In Vitro Evaluation of Tablet Dosage Form of Unani Anti-Diabetic Powder Containing Gymnema sylvestre, Syzygium cuminii and Zingiber officinale. Pharmacognosy Research, 15(3), 566-577 (2023) https://doi.org/10.5530/pres.15.3.060.
Dolas RT.Formulation development and evaluation of orodispersible tablet of Azelnidipine by factorial design and its comparison with the marketed formulation. Particulate Science and Technology, 41(7), 1053–1061(2023) https://doi.org/10.1080/02726351.2023.2173109.
Published
How to Cite
Issue
Section
Copyright (c) 2025 Ganesh Shinde, Ravindra Jadhav, Rahul Godge, Dattaprasad Vikhe, Vishal Tambe, Shubham Khule

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
















