Formulation development and evaluation of oil-based PLGA nanocarriers of fluticasone propionate
DOI:
https://doi.org/10.69857/joapr.v13i4.969Keywords:
Corticosteroid formulation, Fluticasone propionate, Nanocarriers, Oil-based formulation, Topical formulationAbstract
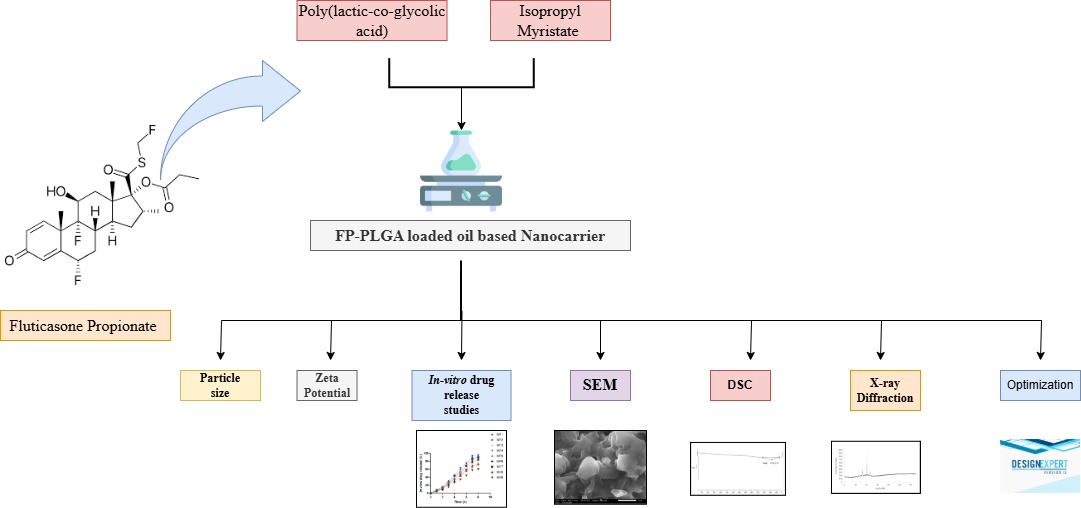
Background: Fluticasone Propionate (FP), a potent corticosteroid, suffers from poor aqueous solubility and limited skin permeability, which reduces its clinical efficacy in topical applications. This work aims to overcome these limitations; oil-based poly(lactic-co-glycolic acid) (PLGA) nanocarriers were developed to enhance the solubility, stability, and sustained release of FP. Methodology: A 3² factorial design was employed to formulate nine batches of PLGA nanocarriers loaded with FP using varying concentrations of PLGA and Capmul MCM. The formulations were evaluated for particle size, zeta potential, drug content, and in vitro drug release. The optimized batch was further characterized using Scanning Electron Microscopy (SEM), Differential Scanning Calorimetry (DSC), and X-Ray Diffraction (XRD). Stability studies were conducted over 30 days under accelerated conditions. Results and Discussion: Among all batches, formulation F1 exhibited optimal characteristics, with a particle size of 197.5 nm, a zeta potential of -27.4 mV, and a drug content of 99.85%. The in vitro drug release profile showed a sustained release of 97% over 12 hours. SEM confirmed a spherical morphology with uniform distribution, while DSC and XRD analyses indicated the amorphous dispersion of the drug within the PLGA matrix. The formulation remained physically and chemically stable during the 30-day accelerated stability testing. Conclusion: The study demonstrates that oil-based PLGA nanocarriers effectively enhance the solubility and controlled delivery of Fluticasone Propionate. Although in vivo validation is pending, the system offers promising potential for improving topical corticosteroid therapy in clinical settings. The novelty of this formulation lies in the strategic combination of Isopropyl Myristate and PLGA to create an oil-based nanocarrier platform, which has not been previously reported for Fluticasone Propionate. This approach enables superior drug encapsulation, enhanced skin permeability, and controlled drug delivery.
Downloads
References
Amasya G, Şengel Türk CT, Badilli U, Tarimci N. Development and Statistical Optimization of Solid Lipid Nanoparticle Formulations of Fluticasone Propionate. Turkish J. Pharm. Sci., 17, 359–66 (2020) https://doi.org/10.4274/tjps.galenos.2019.27136.
Syed YY. Fluticasone Furoate/Vilanterol: a Review of Its Use in Patients with Asthma. Drugs, 75, 407–18 (2015) https://doi.org/10.1007/s40265-015-0354-5.
Yadav S, Jain V, Magar H, Kumar MV, Warde S, Singh RM. A Novel RP-HPLC Method for Simultaneous Estimation of Vilanterol Trifenatate, Umeclidinium Bromide and Fluticasone Furoate in Inhalation Dry Powder Formulation. J. Chromatogr. Sci., 62, 761–6 (2024) https://doi.org/10.1093/chromsci/bmad075.
Johnson M. Development of fluticasone propionate and comparison with other inhaled corticosteroids. J. Allergy Clin. Immunol., 101, S434–9 (1998) https://doi.org/10.1016/S0091-6749(98)70155-1.
Giavina-Bianchi P. Fluticasone furoate nasal spray in the treatment of allergic rhinitis. Ther. Clin. Risk Manag., 4, 465–72 (2008) https://doi.org/10.2147/TCRM.S1984.
Elsisi R, Helal D, Mekhail G, Abou Hussein D, Osama A. Advancements in Skin Aging Treatment: Exploring Antioxidants and Nanoparticles for Enhanced Skin Permeation. Arch. Pharm. Sci. Ain Shams Univ., 7, 376–401 (2023) https://doi.org/10.21608/aps.2023.250333.1146.
Tavares Luiz M, Santos Rosa Viegas J, Palma Abriata J, Viegas F, Testa Moura de Carvalho Vicentini F, Lopes Badra Bentley MV, Chorilli M, Maldonado Marchetti J, Tapia-Blácido DR. Design of experiments (DoE) to develop and to optimize nanoparticles as drug delivery systems. Eur. J. Pharm. Biopharm., 165, 127–48 (2021) https://doi.org/10.1016/j.ejpb.2021.05.011.
Tang C, Niu X, Shi L, Zhu H, Lin G, Xu R. In vivo Pharmacokinetic Drug-Drug Interaction Studies Between Fedratinib and Antifungal Agents Based on a Newly Developed and Validated UPLC/MS-MS Method. Front. Pharmacol., 11, (2021) https://doi.org/10.3389/fphar.2020.626897.
Ansari MJ, Alnakhli M, Al-Otaibi T, Meanazel O Al, Anwer MK, Ahmed MM, et al. Formulation and evaluation of self-nanoemulsifying drug delivery system of brigatinib: Improvement of solubility, in vitro release, ex-vivo permeation and anticancer activity. J. Drug Deliv. Sci. Technol., 61, 102204 (2021) https://doi.org/10.1016/j.jddst.2020.102204.
Rane BR, Jain AS, Mane NP, Patil V, Patil MS, Bavaskar KR. Fabrication and Evaluation of Carbocisteine-Loaded Solid Lipid Nanoparticles To Treat Pulmonary Infections. J. Appl. Pharm. Res., 12, 122–36 (2024) https://doi.org/10.69857/joapr.v12i6.661.
Jojo GM, Kuppusamy G, De A, Karri VVSNR. Formulation and optimization of intranasal nanolipid carriers of pioglitazone for the repurposing in Alzheimer’s disease using Box-Behnken design. Drug Dev. Ind. Pharm., 45, 1061–72 (2019) https://doi.org/10.1080/03639045.2019.1593439.
Pawar SK, Vavia PR. Rice germ oil as multifunctional excipient in preparation of self-microemulsifying drug delivery system (SMEDDS) of tacrolimus. AAPS PharmSciTech, 13, 254–61 (2012) https://doi.org/10.1208/s12249-011-9748-1.
Elmowafy M, Al-Sanea MM. Nanostructured lipid carriers (NLCs) as drug delivery platform: Advances in formulation and delivery strategies. Saudi Pharm. J., 29, 999–1012 (2021) https://doi.org/10.1016/j.jsps.2021.07.015.
Tamjidi F, Shahedi M, Varshosaz J, Nasirpour A. Nanostructured lipid carriers (NLC): A potential delivery system for bioactive food molecules. Innov. Food Sci. Emerg. Technol., 19, 29–43 (2013) https://doi.org/10.1016/j.ifset.2013.03.002.
Leng D, Thanki K, Fattal E, Foged C, Yang M. Engineering of budesonide-loaded lipid-polymer hybrid nanoparticles using a quality-by-design approach. Int. J. Pharm., 548, 740–6 (2018) https://doi.org/10.1016/j.ijpharm.2017.08.094.
Hemnani N, Suresh PK. Fabrication and study of release kinetics of moxifloxacin and dexamethasone loaded nanostructured lipid carrier system for ocular drug delivery. J. Appl. Pharm. Res., 13, 141–53 (2025) https://doi.org/10.69857/joapr.v13i3.1162.
Yin J, Hou Y, Song X, Wang P, Li Y. Cholate-modified polymer-lipid hybrid nanoparticles for oral delivery of quercetin to potentiate the antileukemic effect. Int. J. Nanomedicine, 14, 4045–57 (2019) https://doi.org/10.2147/IJN.S210057.
Pawar MA, Shevalkar GB, Vavia PR. Design and Development of Gastro-retentive Drug Delivery System for Trazodone Hydrochloride: a Promising Alternative to Innovator’s Controlled-Release Tablet. AAPS PharmSciTech, 23, 251 (2022) https://doi.org/10.1208/s12249-022-02404-8.
Shevalkar G, Pawar M, Vavia P. Nanostructured Lipid Carriers (NLCs) of Lumefantrine with Enhanced Permeation. J. Pharm. Innov., 17, 1221–34 (2022) https://doi.org/10.1007/s12247-021-09590-1.
Thete R, Shevalkar G, Borse L. Development of Nanostructured Lipid Carriers for Donepezil Hydrochloride Effective Nose to Brain Delivery. Biosci. Biotechnol. Res. Asia, 21, 1145–56 (2024) https://doi.org/10.13005/bbra/3293.
Darandale SS, Shevalkar GB, Vavia PR. Effect of Lipid Composition in Propofol Formulations: Decisive Component in Reducing the Free Propofol Content and Improving Pharmacodynamic Profiles. AAPS PharmSciTech, 18, 441–50 (2017) https://doi.org/10.1208/s12249-016-0524-0.
Shevalkar G, Vavia P. Solidified nanostructured lipid carrier (S-NLC) for enhancing the oral bioavailability of ezetimibe. J. Drug Deliv. Sci. Technol., 53, 101211 (2019) https://doi.org/10.1016/j.jddst.2019.101211.
Das S, Chaudhury A. Recent Advances in Lipid Nanoparticle Formulations with Solid Matrix for Oral Drug Delivery. AAPS PharmSciTech, 12, 62–76 (2011) https://doi.org/10.1208/s12249-010-9563-0.
Nunse D, Shevalkar GB, Borse L. Innovative Polymeric Micelles with In-Situ Gelation for Enhanced Ocular Delivery of Ketoconazole. J. Pharm. Innov., 20, 1–12 (2025) https://doi.org/10.1007/s12247-024-09915-w.
Shevalkar G, Borse L. Self-microemulsifying drug delivery system (SMEDDS) for oral delivery of zafirlukast: Design, formulation, and pharmacokinetic evaluation. J. Drug Deliv. Sci. Technol., 101, 106298 (2024) https://doi.org/10.1016/j.jddst.2024.106298.
Shevalkar G, Pai R, Vavia P. Nanostructured Lipid Carrier of Propofol: a Promising Alternative to Marketed Soybean Oil–Based Nanoemulsion. AAPS PharmSciTech, 20, 201 (2019) https://doi.org/10.1208/s12249-019-1408-x.
Published
How to Cite
Issue
Section
Copyright (c) 2025 Aniruddha Shejwal, Ganesh B. Shevalkar, Laxmikant B. Borse

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
















