Applications of bioactive compounds of traditional Chinese medicine in breast cancer management
DOI:
https://doi.org/10.69857/joapr.v13i4.935Keywords:
Breast Cancer, Chinese herbs, Phytomedicine, Bioactive compounds, Bioinformatics tools, Traditional Chinese Medicine, TCM databasesAbstract
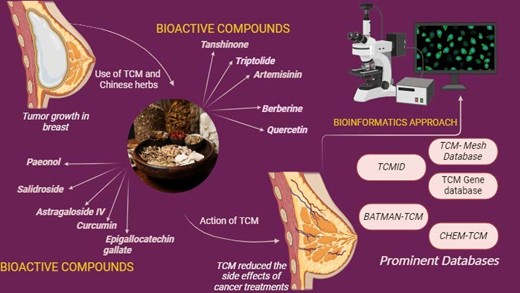
Background: Over the past few decades, the prevalence of breast cancer has been rapidly increasing, making it one of the most prevalent malignancies diagnosed in women globally. Traditional Chinese Medicine (TCM) has gained attention as a potential approach for managing breast cancer by boosting immune response, inhibiting cancer-related gene activity, and alleviating the adverse effects of radiotherapy and chemotherapy. TCM offers a valuable framework for therapeutic systems and scientific exploration that is widely practiced in many regions worldwide, primarily in China, Korea, and Japan. The herbal components of TCM exhibit complex biological activities that influence multiple aspects of cancer progression, including cell proliferation, programmed cell death (apoptosis), immune modulation, and tumor-host interactions. Methodology: A systematic literature review was conducted using peer-reviewed articles published between 2017 and 2024. Relevant data were collected from publicly available scientific databases. Non-English, Conference papers, and duplicate studies were excluded to ensure the inclusion of high-quality and relevant research findings. Result and Discussion: Analysis revealed that specific bioactive compounds in TCM exhibit significant anti-cancer effects. For example, ginsenoside Rg3 inhibited tumor growth by 45% in vivo, while curcumin reduced MDA-MB-231 breast cancer cell viability by 60% at 20 μM. Conclusion: The promise of TCM, especially its bioactive components and medicinal herbs in the treatment of breast cancer, is the main highlight of this paper. Additionally, it highlights the key scientific databases that provide critical insights into TCM research while exploring the therapeutic mechanisms of Chinese herbs and their bioactive components in mitigating breast cancer progression.
Downloads
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clinicians, 68, 394–424 (2018) https://doi.org/10.3322/caac.21492.
Diao X, Guo C, Jin Y, Li B, Gao X, Du X, Chen Z, Jo M, Zeng Y, Ding C, Liu W, Guo J, Li S, Qiu H. Cancer situation in China: an analysis based on the global epidemiological data released in 2024. Cancer Communications, 45, 178–97 (2024) https://doi.org/10.1002/cac2.12627
Lei S, Zheng R, Zhang S, Wang S, Chen R, Sun K, Zeng H, Zhou J, Wei W. Global patterns of breast cancer incidence and mortality: A population‐based cancer registry data analysis from 2000 to 2020. Cancer Communications, 41, 1183–94 (2021) https://doi.org/10.1002/cac2.12207.
Cao W, Chen H-D, Yu Y-W, Li N, Chen W-Q. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chinese Medical Journal, 134, 783–91 (2021) https://doi.org/10.1097/cm9.0000000000001474
Feng R-Q, Li D-H, Liu X-K, Zhao X-H, Wen Q-E, Yang Y. Traditional Chinese Medicine for Breast Cancer: A Review. BCTT, Volume 15, 747–59 (2023) https://doi.org/10.2147/bctt.s429530.
Waks AG, Winer EP. Breast Cancer Treatment. JAMA, 321, 288 (2019) https://doi.org/10.1001/jama.2018.19323.
Spronk I, Schellevis FG, Burgers JS, de Bock GH, Korevaar JC. Incidence of isolated local breast cancer recurrence and contralateral breast cancer: A systematic review. The Breast, 39, 70–9 (2018) https://doi.org/10.1016/j.breast.2018.03.011.
Coughlin SS. Epidemiology of Breast Cancer in Women, Springer International Publishing, 9-29 (2019) https://doi.org/10.1007/978-3-030-20301-6_2.
Akram M, Iqbal M, Daniyal M, Khan AU. Awareness and current knowledge of breast cancer. Biol Res, 50, (2017) https://doi.org/10.1186/s40659-017-0140-9.
Zhang X, Qiu H, Li C, Cai P, Qi F. The positive role of traditional Chinese medicine as an adjunctive therapy for cancer. BST, 15, 283–98 (2021) https://doi.org/10.5582/bst.2021.01318.
Wang Y, Zhang Q, Chen Y, Liang C-L, Liu H, Qiu F, Dai Z. Antitumor effects of immunity-enhancing traditional Chinese medicine. Biomedicine & Pharmacotherapy, 121, 109570 (2020) https://doi.org/10.1016/j.biopha.2019.109570.
Zia FZ, Olaku O, Bao T, Berger A, Deng G, Yin Fan A, Garcia MK, Herman PM, Kaptchuk TJ, Ladas EJ, Langevin HM, Lao L, Lu W, Napadow V, Niemtzow RC, Vickers AJ, Shelley Wang X, Witt CM, Mao JJ. The National Cancer Institute’s Conference on Acupuncture for Symptom Management in Oncology: State of the Science, Evidence, and Research Gaps. JNCI Monographs, (2017) https://doi.org/10.1093/jncimonographs/lgx005.
Yiming ZH, Xinchen TI, Yufei WA, Haochen WA, Heran ZH, Yonghong WA, Shulong JI. Exploring traditional Chinese medicine-based diagnosis and treatment of breast cancer based on molecular typing. Journal of Beijing University of Traditional Chinese Medicine, 1, 46(7), (2023) https://doi.org/10.3969/j.issn.1006-2157.2023.07.022.
Guo Q, Coyle ME, Zhang AL, Xue X, Bian W, Song A, Xie X, Hong R, Lyu G, Liu L, Chen Q, Xue CC. Chinese Medicine Syndrome Differentiation for Early Breast Cancer: A Multicenter Prospective Clinical Study. Front. Oncol., 12, (2022) https://doi.org/10.3389/fonc.2022.914805.
Fang Z, Zhang M, Liu J, Zhao X, Zhang Y, Fang L. Tanshinone IIA: A Review of its Anticancer Effects. Front. Pharmacol., 11, (2021) https://doi.org/10.3389/fphar.2020.611087.
Jin Z, Chenghao Y, Cheng P. Anticancer Effect of Tanshinones on Female Breast Cancer and Gynecological Cancer. Front. Pharmacol., 12, (2022) https://doi.org/10.3389/fphar.2021.824531.
Ye T, Xiong D, Chen L, Li Y, Gong S, Zhang L, Li B, Pan J, Qian J, Qu H. Effect of Danshen on TLR2-triggered inflammation in macrophages. Phytomedicine, 70, 153228 (2020) https://doi.org/10.1016/j.phymed.2020.153228.
Zhu P-C, Shen J, Qian R-Y, Xu J, Liu C, Hu W-M, Zhang Y, Lv L-C. Effect of tanshinone IIA for myocardial ischemia/reperfusion injury in animal model: preclinical evidence and possible mechanisms. Front. Pharmacol., 14, (2023) https://doi.org/10.3389/fphar.2023.1165212.
Wu Y-T, Xie L-P, Hua Y, Xu H-L, Chen G-H, Han X, Tan Z-B, Fan H-J, Chen H-M, Li J, Liu B, Zhou Y-C. Tanshinone I Inhibits Oxidative Stress–Induced Cardiomyocyte Injury by Modulating Nrf2 Signaling. Front. Pharmacol., 12, (2021) https://doi.org/10.3389/fphar.2021.644116.
Feng J, Liu L, Yao F, Zhou D, He Y, Wang J. The protective effect of tanshinone IIa on endothelial cells: a generalist among clinical therapeutics. Expert Review of Clinical Pharmacology, 14, 239–48 (2021) https://doi.org/10.1080/17512433.2021.1878877.
Lu Y, Yan Y, Liu X. Effects of alprostadil combined with tanshinone IIa injection on microcirculation disorder, outcomes, and cardiac function in AMI patients after PCI. Ann Palliat Med, 10, 97–103 (2021) https://doi.org/10.21037/apm-20-2147.
Wen J, Chang Y, Huo S, Li W, Huang H, Gao Y, Lin H, Zhang J, Zhang Y, Zuo Y, Cao X, Zhong F. Tanshinone IIA attenuates atherosclerosis via inhibiting NLRP3 inflammasome activation. Aging, 13, 910–32 (2020) https://doi.org/10.18632/aging.202202.
Wang X, Wang W-M, Han H, Zhang Y, Liu J-L, Yu J-Y, Liu H-M, Liu X-T, Shan H, Wu S-C. Tanshinone IIA protected against lipopolysaccharide-induced brain injury through the protective effect of the blood–brain barrier and the suppression of oxidant stress and inflammatory response. Food Funct., 13, 8304–12 (2022) https://doi.org/10.1039/d2fo00710j.
Qin C, Liu S, Zhou S, Xia X, Hu J, Yu Y, Ma D. Tanshinone IIA promotes vascular normalization and boosts Sorafenib’s anti-hepatoma activity via modulating the PI3K-AKT pathway. Front. Pharmacol., 14, (2023) https://doi.org/10.3389/fphar.2023.1189532.
Feng F, Cheng P, Xu S, Li N, Wang H, Zhang Y, Wang W. Tanshinone IIA attenuates silica-induced pulmonary fibrosis via Nrf2-mediated inhibition of EMT and TGF-β1/Smad signaling. Chemico-Biological Interactions, 319, 109024 (2020) https://doi.org/10.1016/j.cbi.2020.109024.
Chen H, Shu H, Su W, Li B, Zhang H, Li L, Lin C, Yi W, Zhan X-Y, Chen C, Li X, Yang Y, Zhou M, Yang M. Tanshinone IIA Has a Potential Therapeutic Effect on Kawasaki Disease and Suppresses Megakaryocytes in Rabbits With Immune Vasculitis. Front. Cardiovasc. Med., 9, (2022) https://doi.org/10.3389/fcvm.2022.873851.
Liu Q, Wang W, Li F, Yu D, Xu C, Hu H. Triptolide Inhibits Breast Cancer Cell Metastasis Through Inducing the Expression of miR-146a, a Negative Regulator of Rho GTPase. oncol res, 27, 1043–50 (2019) https://doi.org/10.3727/096504019x15560124931900.
Xu C, Zhang H, Mu L, Yang X. Artemisinins as Anticancer Drugs: Novel Therapeutic Approaches, Molecular Mechanisms, and Clinical Trials. Front. Pharmacol., 11, (2020) https://doi.org/10.3389/fphar.2020.529881.
von Hagens C, Walter-Sack I, Goeckenjan M, Storch-Hagenlocher B, Sertel S, Elsässer M, Remppis BA, Munzinger J, Edler L, Efferth T, Schneeweiss A, Strowitzki T. Long-term add-on therapy (compassionate use) with oral artesunate in patients with metastatic breast cancer after participating in a phase I study (ARTIC M33/2). Phytomedicine, 54, 140–8 (2019) https://doi.org/10.1016/j.phymed.2018.09.178.
Zhong X-D, Chen L-J, Xu X-Y, Liu Y-J, Tao F, Zhu M-H, Li C-Y, Zhao D, Yang G-J, Chen J. Berberine as a potential agent for breast cancer therapy. Front. Oncol., 12, (2022) https://doi.org/10.3389/fonc.2022.993775.
Jeong J, An JY, Kwon YT, Rhee JG, Lee YJ. Effects of low dose quercetin: Cancer cell‐specific inhibition of cell cycle progression. J. Cellular Biochemistry, 106, 73–82 (2008) https://doi.org/10.1002/jcb.21977.
Latif S, Choi S-H, Gyawali A, Hyeon SJ, Kang Y-S, Ryu H. Antioxidant and Neuroprotective Effects of Paeonol against Oxidative Stress and Altered Carrier-Mediated Transport System on NSC-34 Cell Lines. Antioxidants, 11, 1392 (2022) https://doi.org/10.3390/antiox11071392.
Cai M, Shao W, Yu H, Hong Y, Shi L. <p>Paeonol Inhibits Cell Proliferation, Migration and Invasion and Induces Apoptosis in Hepatocellular Carcinoma by Regulating miR-21-5p/KLF6 Axis</p> CMAR, Volume 12, 5931–43 (2020) https://doi.org/10.2147/cmar.s254485.
Marín V, Burgos V, Pérez R, Maria DA, Pardi P, Paz C. The Potential Role of Epigallocatechin-3-Gallate (EGCG) in Breast Cancer Treatment. IJMS, 24, 10737 (2023) https://doi.org/10.3390/ijms241310737.
Sun A-Q, Ju X-L. Inhibitory effects of salidroside on MCF-7 breast cancer cells in vivo. J Int Med Res, 48, (2020) https://doi.org/10.1177/0300060520968353.
Xia D, Li W, Tang C, Jiang J. Astragaloside IV, as a potential anticancer agent. Front. Pharmacol., 14, (2023) https://doi.org/10.3389/fphar.2023.1065505.
Li L, Li G, Chen M, Cai R. Astragaloside IV enhances the sensibility of lung adenocarcinoma cells to bevacizumab by inhibiting autophagy. Drug Development Research, 83, 461–9 (2021) https://doi.org/10.1002/ddr.21878.
Ye Q, Su L, Chen D, Zheng W, Liu Y. Astragaloside IV Induced miR-134 Expression Reduces EMT and Increases Chemotherapeutic Sensitivity by Suppressing CREB1 Signaling in Colorectal Cancer Cell Line SW-480. Cell Physiol Biochem, 43, 1617–26 (2017) https://doi.org/10.1159/000482025.
Zheng Y, Dai Y, Liu W, Wang N, Cai Y, Wang S, Zhang F, Liu P, Chen Q, Wang Z. Astragaloside IV enhances taxol chemosensitivity of breast cancer via caveolin‐1‐targeting oxidant damage. Journal Cellular Physiology, 234, 4277–90 (2018) https://doi.org/10.1002/jcp.27196.
Tomeh MA, Hadianamrei R, Zhao X. A Review of Curcumin and Its Derivatives as Anticancer Agents. IJMS, 20, 1033 (2019) https://doi.org/10.3390/ijms20051033.
Alibeiki F, Jafari N, Karimi M, Peeri Dogaheh H. Potent anti-cancer effects of less polar Curcumin analogues on gastric adenocarcinoma and esophageal squamous cell carcinoma cells. Sci Rep, 7, (2017) https://doi.org/10.1038/s41598-017-02666-4.
Wei G, Zhang G, Li M, Zheng Y, Zheng W, Wang B, et al.. Panax notoginseng: panoramagram of phytochemical and pharmacological properties, biosynthesis, and regulation and production of ginsenosides. Horticulture Research, 11, (2024) https://doi.org/10.1093/hr/uhae170.
Ratan ZA, Haidere MF, Hong YH, Park SH, Lee J-O, Lee J, Cho JY. Pharmacological potential of ginseng and its major component ginsenosides. Journal of Ginseng Research, 45, 199–210 (2021) https://doi.org/10.1016/j.jgr.2020.02.004.
Zhang P, Liu W, Wang Y. The mechanisms of tanshinone in the treatment of tumors. Front. Pharmacol., 14, (2023) https://doi.org/10.3389/fphar.2023.1282203.
Liu Q, Wang W, Li F, Yu D, Xu C, Hu H. Triptolide Inhibits Breast Cancer Cell Metastasis Through Inducing the Expression of miR-146a, a Negative Regulator of Rho GTPase. oncol res, 27, 1043–50 (2019) https://doi.org/10.3727/096504019x15560124931900.
Xu C, Zhang H, Mu L, Yang X. Artemisinins as Anticancer Drugs: Novel Therapeutic Approaches, Molecular Mechanisms, and Clinical Trials. Front. Pharmacol., 11, (2020) https://doi.org/10.3389/fphar.2020.529881.
Zhong X-D, Chen L-J, Xu X-Y, Liu Y-J, Tao F, Zhu M-H, Li C-Y, Zhao D, Yang G-J, Chen J. Berberine as a potential agent for breast cancer therapy. Front. Oncol., 12, (2022) https://doi.org/10.3389/fonc.2022.993775.
Ezzati M, Yousefi B, Velaei K, Safa A. A review on anti-cancer properties of Quercetin in breast cancer. Life Sciences, 248, 117463 (2020) https://doi.org/10.1016/j.lfs.2020.117463.
Chang X, Feng X, Du M, Li S, Wang J, Wang Y, Liu P. Pharmacological effects and mechanisms of paeonol on antitumor and prevention of side effects of cancer therapy. Front. Pharmacol., 14, (2023) https://doi.org/10.3389/fphar.2023.1194861.
Marín V, Burgos V, Pérez R, Maria DA, Pardi P, Paz C. The Potential Role of Epigallocatechin-3-Gallate (EGCG) in Breast Cancer Treatment. IJMS, 24, 10737 (2023) https://doi.org/10.3390/ijms241310737.
Sun A-Q, Ju X-L. Inhibitory effects of salidroside on MCF-7 breast cancer cells in vivo. J Int Med Res, 48, (2020) https://doi.org/10.1177/0300060520968353.
Ye Q, Su L, Chen D, Zheng W, Liu Y. Astragaloside IV Induced miR-134 Expression Reduces EMT and Increases Chemotherapeutic Sensitivity by Suppressing CREB1 Signaling in Colorectal Cancer Cell Line SW-480. Cell Physiol Biochem, 43, 1617–26 (2017) https://doi.org/10.1159/000482025.
Song X, Zhang M, Dai E, Luo Y. Molecular targets of curcumin in breast cancer (Review). Mol Med Report, (2018) https://doi.org/10.3892/mmr.2018.9665.
Deng X, Wang J, Lu C, Zhou Y, Shen L, Ge A, Fan H, Liu L. Updating the therapeutic role of ginsenosides in breast cancer: a bibliometrics study to an in-depth review. Front. Pharmacol., 14, (2023) https://doi.org/10.3389/fphar.2023.1226629.
Expansion of the Gene Ontology knowledgebase and resources. Nucleic Acids Res, 45, D331–8 (2016) https://doi.org/10.1093/nar/gkw1108.
Li Y, Wang J, Lin F, Yang Y, Chen S-S. A Methodology for Cancer Therapeutics by Systems Pharmacology-Based Analysis: A Case Study on Breast Cancer-Related Traditional Chinese Medicines. PLoS ONE, 12, e0169363 (2017) https://doi.org/10.1371/journal.pone.0169363.
Wang Y, Liu M, Jafari M, Tang J. A critical assessment of Traditional Chinese Medicine databases as a source for drug discovery. Front. Pharmacol., 15, (2024) https://doi.org/10.3389/fphar.2024.1303693.
Song Z, Chen G, Chen CY-C. AI empowering traditional Chinese medicine? Chem. Sci., 15, 16844–86 (2024) https://doi.org/10.1039/d4sc04107k.
Fang Y-C, Huang H-C, Chen H-H, Juan H-F. TCMGeneDIT: a database for associated traditional Chinese medicine, gene and disease information using text mining. BMC Complement Altern Med, 8, (2008) https://doi.org/10.1186/1472-6882-8-58.
Liu Z, Guo F, Wang Y, Li C, Zhang X, Li H, Diao L, Gu J, Wang W, Li D, He F. BATMAN-TCM: a Bioinformatics Analysis Tool for Molecular mechANism of Traditional Chinese Medicine. Sci Rep, 6, (2016) https://doi.org/10.1038/srep21146 .
Zhai Y, Liu L, Zhang F, Chen X, Wang H, Zhou J, Chai K, Liu J, Lei H, Lu P, Guo M, Guo J, Wu J. Network pharmacology: a crucial approach in traditional Chinese medicine research. Chin Med, 20, (2025) https://doi.org/10.1186/s13020-024-01056-z .
Miao R, Meng Q, Wang C, Yuan W. Bibliometric Analysis of Network Pharmacology in Traditional Chinese Medicine. Evidence-Based Complementary and Alternative Medicine, 2022, 1–11 (2022) https://doi.org/10.1155/2022/1583773
Xu T, Chen W, Zhou J, Dai J, Li Y, Zhao Y. NPBS database: a chemical data resource with relational data between natural products and biological sources. Database, 2020, (2020) https://doi.org/10.1093/database/baaa102. .
Wang X, Wang Z-Y, Zheng J-H, Li S. TCM network pharmacology: A new trend towards combining computational, experimental and clinical approaches. Chinese Journal of Natural Medicines, 19, 1–11 (2021) https://doi.org/10.1016/s1875-5364(21)60001-8.
Li S, Wan F, Shu H, Jiang T, Zhao D, Zeng J. MONN: A Multi-objective Neural Network for Predicting Compound-Protein Interactions and Affinities. Cell Systems, 10, 308-322.e11 (2020) https://doi.org/10.1016/j.cels.2020.03.002.
Zhang L, Chen W-X, Li L-L, Cao Y-Z, Geng Y-D, Feng X-J, Wang A-Y, Chen Z-L, Lu Y, Shen A-Z. Paeonol Suppresses Proliferation and Motility of Non-Small-Cell Lung Cancer Cells by Disrupting STAT3/NF-κB Signaling. Front. Pharmacol., 11, (2020) https://doi.org/10.3389/fphar.2020.572616 .
Ding Q, Hou S, Zu S, Zhang Y, Li S. VISAR: an interactive tool for dissecting chemical features learned by deep neural network QSAR models. Bioinformatics, 36, 3610–2 (2020) https://doi.org/10.1093/bioinformatics/btaa187.
Öztürk H, Özgür A, Ozkirimli E. DeepDTA: deep drug–target binding affinity prediction. Bioinformatics, 34, i821–9 (2018) https://doi.org/10.1093/bioinformatics/bty593.
Karimi M, Wu D, Wang Z, Shen Y. DeepAffinity: interpretable deep learning of compound–protein affinity through unified recurrent and convolutional neural networks. Bioinformatics, 35, 3329–38 (2019) https://doi.org/10.1093/bioinformatics/btz111.
Cheng F, Desai RJ, Handy DE, Wang R, Schneeweiss S, Barabási A-L, Loscalzo J. Network-based approach to prediction and population-based validation of in silico drug repurposing. Nat Commun, 9, (2018) https://doi.org/10.1038/s41467-018-05116-5.
Xu H-Y, Zhang Y-Q, Liu Z-M, Chen T, Lv C-Y, Tang S-H, Zhang X-B, Zhang W, Li Z-Y, Zhou R-R, Yang H-J, Wang X-J, Huang L-Q. ETCM: an encyclopaedia of traditional Chinese medicine. Nucleic Acids Research, 47, D976–82 (2018) https://doi.org/10.1093/nar/gky987.
Wu Y, Zhang F, Yang K, Fang S, Bu D, Li H, Sun L, Hu H, Gao K, Wang W, Zhou X, Zhao Y, Chen J. SymMap: an integrative database of traditional Chinese medicine enhanced by symptom mapping. Nucleic Acids Research, 47, D1110–7 (2018) https://doi.org/10.1093/nar/gky1021.
Guo J-C, Zhang P, Zhou L, You L, Liu Q-F, Zhang Z-G, Sun B, Liang Z-Y, Lu J, Yuan D, Tan A-D, Sun J, Liao Q, Dai M-H, Xiao GG, Li S, Zhang T-P. Prognostic and predictive value of a five-molecule panel in resected pancreatic ductal adenocarcinoma: A multicentre study. eBioMedicine, 55, 102767 (2020) https://doi.org/10.1016/j.ebiom.2020.102767.
Kong X, Liu C, Zhang Z, Cheng M, Mei Z, Li X, Liu P, Diao L, Ma Y, Jiang P, Kong X, Nie S, Guo Y, Wang Z, Zhang X, Wang Y, Tang L, Guo S, Liu Z, Li D. BATMAN-TCM 2.0: an enhanced integrative database for known and predicted interactions between traditional Chinese medicine ingredients and target proteins. Nucleic Acids Research, 52, D1110–20 (2023) https://doi.org/10.1093/nar/gkad926.
N B. Network pharmacology based investigation on the mechanism of tetrandrine against breast cancer. Phytomedicine Plus, 3, 100381 (2023) https://doi.org/10.1016/j.phyplu.2022.100381.
Hua H, Tang J-Y, Zhao J-N, Wang T, Zhang J-H, Yu J-Y, Yang C-M, Ai Y-L, Luo Q-X. From traditional medicine to modern medicine: the importance of TCM regulatory science (TCMRS) as an emerging discipline. Chin Med, 20, (2025) https://doi.org/10.1186/s13020-025-01152-8.
Huang N, Huang W, Wu J, Long S, Luo Y, Huang J. Possible opportunities and challenges for traditional Chinese medicine research in 2035. Front. Pharmacol., 15, (2024) https://doi.org/10.3389/fphar.2024.1426300.
Zhu L, Mou W, Lai Y, Lin J, Luo P. Language and cultural bias in AI: comparing the performance of large language models developed in different countries on Traditional Chinese Medicine highlights the need for localized models. J Transl Med, 22, (2024) https://doi.org/10.1186/s12967-024-05128-4.
Liu W, Yang B, Yang L, Kaur J, Jessop C, Fadhil R, Good D, Ni G, Liu X, Mosaiab T, Yi Z, Wei MQ. Therapeutic Effects of Ten Commonly Used Chinese Herbs and Their Bioactive Compounds on Cancers. Evidence-Based Complementary and Alternative Medicine, 2019, 1–10 (2019) https://doi.org/10.1155/2019/6057837.
Almatroodi SA, Alsahli MA, Rahmani AH. Berberine: An Important Emphasis on Its Anticancer Effects through Modulation of Various Cell Signaling Pathways. Molecules, 27, 5889 (2022) https://doi.org/10.3390/molecules27185889.
Fu J, Yu L, Luo J, Huo R, Zhu B. Paeonol induces the apoptosis of the SGC‑7901 gastric cancer cell line by downregulating ERBB2 and inhibiting the NF‑κB signaling pathway. Int J Mol Med, (2018) https://doi.org/10.3892/ijmm.2018.3704.
Deeken JF, Wang H, Hartley M, Cheema AK, Smaglo B, Hwang JJ, He AR, Weiner LM, Marshall JL, Giaccone G, Liu S, Luecht J, Spiegel JY, Pishvaian MJ. A phase I study of intravenous artesunate in patients with advanced solid tumor malignancies. Cancer Chemother Pharmacol, 81, 587–96 (2018) https://doi.org/10.1007/s00280-018-3533-8.
Liu X, Cao J, Huang G, Zhao Q, Shen J. Biological Activities of Artemisinin Derivatives Beyond Malaria. CTMC, 19, 205–22 (2019) https://doi.org/10.2174/1568026619666190122144217.
Varghese E, Samuel SM, Varghese S, Cheema S, Mamtani R, Büsselberg D. Triptolide Decreases Cell Proliferation and Induces Cell Death in Triple Negative MDA-MB-231 Breast Cancer Cells. Biomolecules, 8, 163 (2018) https://doi.org/10.3390/biom8040163.
Sun Y, Zhou Q, Chen F, Gao X, Yang L, Jin X, Wink M, Sharopov FS, Sethi G. Berberine inhibits breast carcinoma proliferation and metastasis under hypoxic microenvironment involving gut microbiota and endogenous metabolites. Pharmacological Research, 193, 106817 (2023) https://doi.org/10.1016/j.phrs.2023.106817.
Maugeri A, Calderaro A, Patanè GT, Navarra M, Barreca D, Cirmi S, Felice MR. Targets Involved in the Anti-Cancer Activity of Quercetin in Breast, Colorectal and Liver Neoplasms. IJMS, 24, 2952 (2023) https://doi.org/10.3390/ijms24032952.
Wang Y, Li B-S, Zhang Z-H, Wang Z, Wan Y-T, Wu F-W, Liu J-C, Peng J-X, Wang H-Y, Hong L. Paeonol repurposing for cancer therapy: From mechanism to clinical translation. Biomedicine & Pharmacotherapy, 165, 115277 (2023) https://doi.org/10.1016/j.biopha.2023.115277.
Banerjee S, Mandal AKA. Role of epigallocatechin-3- gallate in the regulation of known and novel microRNAs in breast carcinoma cells. Front. Genet., 13, (2022) https://doi.org/10.3389/fgene.2022.995046.
Ren M, Xu W, Xu T. Salidroside represses proliferation, migration and invasion of human lung cancer cells through AKT and MEK/ERK signal pathway. Artificial Cells, Nanomedicine, and Biotechnology, 47, 1014–21 (2019) https://doi.org/10.1080/21691401.2019.1584566.
Chen L, Zhuo D, Yuan H. Clinical effect of astragaloside IV on breast carcinoma cells based on MDR1: A randomised trial. Trop. J. Pharm Res, 20, 2311–6 (2021) https://doi.org/10.4314/tjpr.v20i11.12.
Xue R, Fang Z, Zhang M, Yi Z, Wen C, Shi T. TCMID: traditional Chinese medicine integrative database for herb molecular mechanism analysis. Nucleic Acids Research, 41, D1089–95 (2012) https://doi.org/10.1093/nar/gks1100.
Chen CY-C. TCM Database@Taiwan: The World’s Largest Traditional Chinese Medicine Database for Drug Screening In Silico. PLoS ONE, 6, e15939 (2011) https://doi.org/10.1371/journal.pone.0015939.
Published
How to Cite
Issue
Section
Copyright (c) 2025 Saumya Srivastava, Vijay Jagdish Upadhye, Madhulika Esther Prasad, Pallavi Singh

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
















