Design, formulation, and evaluation of hydrogel network based microbeads for prolonged release of valacyclovir
DOI:
https://doi.org/10.69857/joapr.v13i5.1668Keywords:
Valacyclovir hydrochloride, hydrogel microbeads, gellan gum, sodium alginate, ionic gelationAbstract
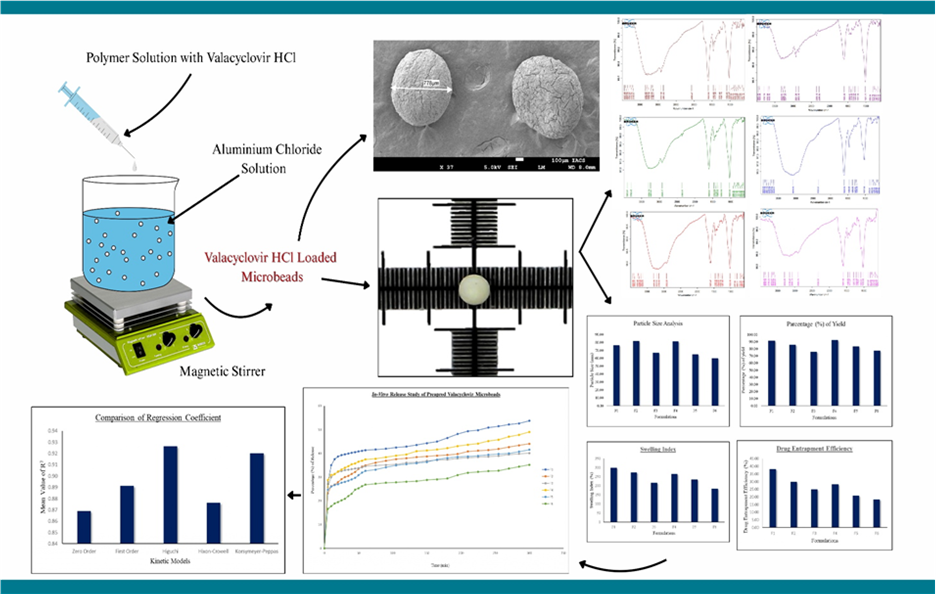
Background: This study aimed to formulate and develop hydrogel network-based microbeads for the prolonged release of the antiviral drug Valacyclovir, focusing on the effect of natural gum/polymer ratios in their preparation. Methodology: Microbeads containing Valacyclovir hydrochloride were prepared using the ionotropic gelation method, which involved sodium alginate and gellan gum as the polymers, and aluminum chloride as the crosslinking agent. Result: In the evaluation, the F1 batch exhibited the highest swelling capacity, drug entrapment efficiency, and drug release profile. Across all formulations, the particles were round to oval in shape, with sizes ranging from 598 to 816µm. Drug release kinetics revealed that the Higuchi model best explained the formulations. The surface morphology of the best-performing formulation was examined using scanning electron microscopy (SEM). Discussion: The findings proposed that the prepared microbeads function as swellable matrix-type systems, enabling prolonged drug delivery. The Higuchi model fit supported a diffusion-controlled release mechanism, while the SEM analysis confirmed the suitability of the microbead structure for sustained release applications. Conclusion: These kinds of ionotropically-gelled alginate-based microbeads may improve patient compliance by reducing dosing frequency and enhancing oral bioavailability.
Downloads
References
Khan Z, Abourehab MAS, Parveen N, Kohli K, Kesharwani P. Recent advances in microbeads-based drug delivery system for achieving controlled drug release. J Biomater Sci Polym Ed, 34(4), 541–564 (2023) https://doi.org/10.1080/09205063.2022.2127237.
Lengyel M, Kállai-Szabó N, Antal V, Laki AJ, Antal I. Microparticles, microspheres, and microcapsules for advanced drug delivery. Sci Pharm, 87(3), 20 (2019) https://doi.org/10.3390/scipharm87030020.
Jarvas G, Szerenyi D, Jankovics H, Vonderviszt F, Tovari J, Takacs L, et al. Microbead-based extracorporeal immuno-affinity virus capture: a feasibility study to address the SARS-CoV-2 pandemic. Microchim Acta, 190(3), 95 (2023) https://doi.org/10.1007/s00604-023-05671-9.
Boi S, Rouatbi N, Dellacasa E, Di Lisa D, Bianchini P, Monticelli O, et al. Alginate microbeads with internal microvoids for the sustained release of drugs. Int J Biol Macromol, 156, 454–461 (2020) https://doi.org/10.1016/j.ijbiomac.2020.04.083.
Li H, Zhou J, Zhou Y, Dao J, Wei D, Wang Y. Advances in photocrosslinked natural hydrogel-based microspheres for bone repair. J Polym Sci, 62(22), 4966–4992 (2024) https://doi.org/10.1002/pol.20240302.
Markovic M, Ben-Shabat S, Dahan A. Prodrugs for improved drug delivery: lessons learned from recently developed and marketed products. Pharmaceutics, 12(11), 1031 (2020) https://doi.org/10.3390/pharmaceutics12111031.
Abdalla S, Briand C, Oualha M, Bendavid M, Béranger A, Benaboud S, et al. Population pharmacokinetics of intravenous and oral acyclovir and oral valacyclovir in pediatric population to optimize dosing regimens. Antimicrob Agents Chemother, 64(12), 01426-20 (2020) https://doi.org/10.1128/aac.01426-20.
Das S, Samanta A, Das S, Nayak AK. Sustained release of acyclovir from alginate-gellan gum and alginate-xanthan gum microbeads. JCIS Open, 13, 100106 (2024) https://doi.org/10.1016/j.jciso.2024.100106.
Kurtulbaş E, Albarri R, Torun M, Şahin S. Encapsulation of Moringa oleifera leaf extract in chitosan-coated alginate microbeads produced by ionic gelation. Food Biosci, 50, 102158 (2022) https://doi.org/10.1016/j.fbio.2022.102158.
Dash S, Gutti P, Behera B, Mishra D. Anionic species from multivalent metal salts are differentially retained during aqueous ionic gelation of sodium alginate and could fine-tune the hydrogel properties. Int J Biol Macromol, 265, 130767 (2024) https://doi.org/10.1016/j.ijbiomac.2024.130767.
Yang D. Recent advances in hydrogels. Chem Mater, 34(5), 1987–1989 (2022) https://doi.org/10.1021/acs.chemmater.2c00188.
Jana S, Pramanik R, Nayak AK, Sen KK. Gellan gum (GG)-based IPN microbeads for sustained drug release. J Drug Deliv Sci Technol, 69, 103034 (2022) https://doi.org/10.1016/j.jddst.2021.103034.
Bhopte DK, Sagar R, Kori ML. Fabrication, optimization and characterization of floating microspheres of quinapril hydrochloride using factorial design method. Biomed Pharmacol J, 15(4), 2011–2024 (2022) https://doi.org/10.13005/bpj/2539.
Khan HU, Nasir F, Maheen S, Shafqat SS, Shah S, Khames A, et al. Antibacterial and wound-healing activities of statistically optimized nitrofurazone- and lidocaine-loaded silica microspheres by the Box–Behnken design. Molecules, 27(8), 2532 (2022) https://doi.org/10.3390/molecules27082532.
Wu MY, Kao IF, Fu CY, Yen SK. Effects of adding chitosan on drug entrapment efficiency and release duration for paclitaxel-loaded hydroxyapatite–gelatin composite microspheres. Pharmaceutics, 15(8), 2025 (2023) https://doi.org/10.3390/pharmaceutics15082025.
Long T, Tan W, Tian X, Tang Z, Hu K, Ge L, et al. Gelatin/alginate-based microspheres with sphere-in-capsule structure for spatiotemporal manipulative drug release in gastrointestinal tract. Int J Biol Macromol, 226, 485–495 (2023) https://doi.org/10.1016/j.ijbiomac.2022.12.040.
Sharma Y, Mahar R, Chakraborty A, Nainwal N. Optimizing the formulation variables for encapsulation of linezolid into polycaprolactone inhalable microspheres using double emulsion solvent evaporation. Tuberculosis, 143, 102417 (2023) https://doi.org/10.1016/j.tube.2023.102417.
Frent OD, Duda-Seiman DM, Vicas LG, Duteanu N, Nemes NS, Pascu B, et al. Study of the influence of the excipients used for the synthesis of microspheres loaded with quercetin: their characterization and antimicrobial activity. Coatings, 13(8), 1376 (2023) https://doi.org/10.3390/coatings13081376.
Frenț OD, Duteanu N, Teusdea AC, Ciocan S, Vicaș L, Jurca T, et al. Preparation and characterization of chitosan-alginate microspheres loaded with quercetin. Polymers, 14(3), 490 (2022) https://doi.org/10.3390/polym14030490.
Mundarinti SHB, Ahad HA. Impact of Pistacia lentiscus plant gum on particle size and swelling index in central composite designed amoxycillin trihydrate mucoadhesive microspheres. Indian J Pharm Educ Res, 57, 763–772 (2023) https://doi.org/10.5530/ijper.57.3.93.
Nandee R, Chowdhury MA, Hossain N, Rana MM, Mobarak MH, Khandaker MR. Surface topography and surface morphology of graphene nanocomposite by FESEM, EDX and AFM analysis. Nano-Structures & Nano-Objects, 38, 101170 (2024) https://doi.org/10.1016/j.nanoso.2023.101170.
Xing L, Ding J, Chen D, Xiong C, Huang T, Xiong Z. Regulation of the surface morphology of microspheres by polymers with different crystallinities. New J Chem, 49(10), 4050–4060 (2025) https://doi.org/10.1039/D4NJ05113K.
Dobhal K, Verma S, Singh A, Kukreti G. Fabrication and evaluation of floating zidovudine microbeads for prolonged kinetic release and bioavailability. Indian Journal of Pharmaceutical Education and Research, 59(1), 65–73 (2024) https://doi.org/10.5530/ijper.20254139.
Das S, Dey R. Trivalent ion cross-linked and acetalated gellan gum microspheres of glimepiride. Asian J Pharm Clin Res, 13(5), 66–68 (2020) https://doi.org/10.22159/ajpcr.2020.v13i5.37229.
Giotopoulou I, Stamatis H, Barkoula NM. Encapsulation of thymol in ethyl cellulose-based microspheres and evaluation of its sustained release for food applications. Polymers, 16(23), 3396 (2024) https://doi.org/10.3390/polym16233396.
Roy D, Das S, Samanta A. Design and in vitro release kinetics of liposomal formulation of acyclovir. Int J Appl Pharm, 11(6), 61–65 (2019) https://doi.org/10.22159/ijap.2019v11i6.34917.
Andaririt DR, Purnaningtyas SRD, Wijayanto A. Validation of the UV-Vis spectrophotometric method for the determination of ascorbic acid content in beverage preparations based on a standard vitamin C calibration curve. Open Access Health Scientific Journal, 6(2), 249–257 (2025) https://doi.org/10.55700/oahsj.v6i2.101.
Mallick A, Sahu R, Nandi G, Dua TK, Shaw TK, Dhar A, et al. Development of liposomal formulation for controlled delivery of valacyclovir: an in vitro study. J Pharm Innov, 18(3), 1020–1029 (2023) https://doi.org/10.1007/s12247-022-09706-1.
Mishra A, Sinha VR, Sharma S, Mathew AT, Kumar R, Yadav AK. Molecular and qualitative characterization of compatibility between valacyclovir hydrochloride and excipients as raw materials for the development of solid oral dosage formulation. American Journal of Biopharmacy and Pharmaceutical Sciences, 3(1), 8–18 (2023) https://doi.org/10.25259/AJBPS_12_2023.
Thombare N, Mahto A, Singh D, Chowdhury AR, Ansari MF. Comparative FTIR characterization of various natural gums: a criterion for their identification. J Polym Environ, 31(8), 3372–3380 (2023) https://doi.org/10.1007/s10924-023-02821-1.
Published
How to Cite
Issue
Section
Copyright (c) 2025 Sankar Narayan Bhunia, Dipankar Saha, Sudipta Das, Rimi Dey, Sawan Das

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
















