A QbD-based stability-indicating RP-HPLC method for larotrectinib: degradation kinetics and integrated white, green, and blue analytical assessment
DOI:
https://doi.org/10.69857/joapr.v13i4.1436Keywords:
Larotrectinib, Quality by Design, RP HPLC, Forced Degradation, Greenness, WhitenessAbstract
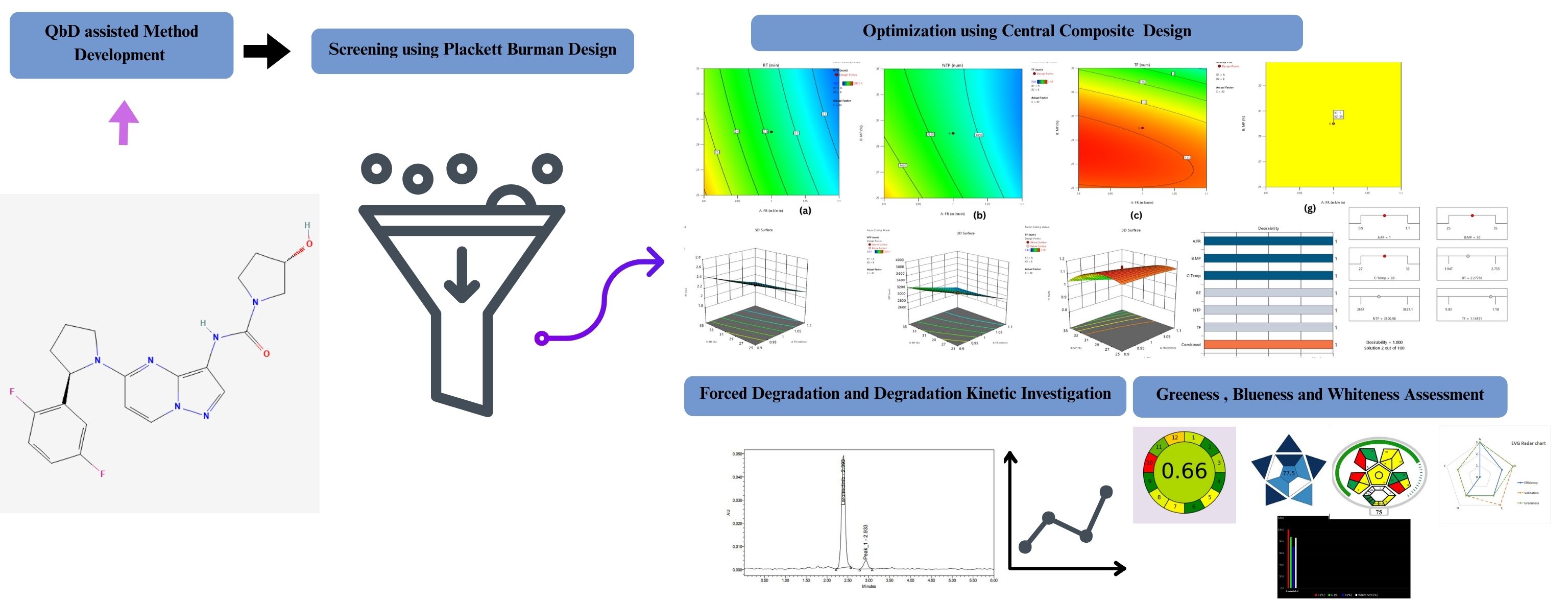
Background: Larotrectinib, a selective TRK inhibitor, received FDA approval on April 10, 2025, for treating solid tumors with NTRK gene fusions. Despite its therapeutic significance, no RP-HPLC method using a Quality-by-Design (QbD) framework has been reported. This study aimed to develop and validate a QbD-based RP-HPLC method for larotrectinib estimation. Methodology: Critical Analytical Parameters (CAPs) were identified using a Plackett–Burman Design and optimized via a Central Composite Design (CCD). Separation was achieved on a Sunfire C18 column (250 × 4.6 mm, 5 µm) with a mobile phase of 0.1% OPA and acetonitrile (70:30, v/v), flow rate 1.0 mL/min, injection volume 10 µL, and detection at 262 nm. Optimized conditions from the Method Operable Design Region (MODR) gave a desirability value of 1. Results and Discussion: The method achieved sharp separation with a retention time of 2.2 min in a 5-minute runtime. Validation per ICH Q2(R1) confirmed linearity (12.5–75 µg/mL, R² = 0.9998), intra- and inter-day precision (%RSD < 2%), mean recovery of 99.29%, and sensitivity with DL 0.30 µg/mL and QL 0.92 µg/mL. Forced degradation studies revealed zero-order kinetics under 0.1 N HCl, 0.5 N NaOH, and thermal stress, and first-order kinetics under 0.5 N HCl, 0.1N NaOH, 3% and 5% H₂O₂, and water. Greenness, blueness, whiteness, and sustainability were assessed using AMGS, AGREE, ComplexMoGAPI, BAGI, RGB, and EVG tools, yielding favourable outcomes. Conclusion: The developed QbD-based RP-HPLC method is robust, validated, and stability-indicating, suitable for quality control, regulatory submissions, and bioanalysis of larotrectinib.
Downloads
References
Bayer. “Approval of Vitrakvi.”: https://www.bayer.com/en/us/news-stories/approval-of-vitrakvi, cited 01 July, 2025.
Waguespack SG, Drilon A, Lin JJ, Brose MS, McDermott R, Almubarak M, Bauman J, Casanova M, Krishnamurthy A, Kummar S, Leyvraz S, Oh D-Y, Park K, Sohal D, Sherman E, Norenberg R, Silvertown JD, Brega N, Hong DS, Cabanillas ME. Efficacy and safety of larotrectinib in patients with TRK fusion-positive thyroid carcinoma. European Journal of Endocrinology, 186, 631–43 (2022) https://doi.org/10.1530/eje-21-1259.
Murciano-Goroff YR, Uppal M, Chen M, Harada G, Schram AM. Basket Trials: Past, Present, and Future. Annual Review of Cancer Biology, 8, 59–80 (2024) https://doi.org/10.1146/annurev-cancerbio-061421-012927.
Bayer. “Bayer to obtain full rights to global development and commercialization of oncology compounds Vitrakvi (larotrectinib) and BAY 2731954 (LOXO 195).” : <https://www.prnewswire.com/news-releases/bayer-to-obtain-full-rights-to-global-development-and-commercialization-of-oncology-compounds-vitrakvi-larotrectinib-and-bay-2731954-loxo-195-300796567.html, cited 23 July, 2025>.
DrugBank. “Larotrectinib – DB14723”: https://go.drugbank.com/drugs/DB14723, cited 23 July, 2025.
U.S. Food and Drug Administration. “Vitrakvi (larotrectinib) prescribing information, 2022.”<https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/210861s008lbl.pdf, cited 23 July, 2025>.
National Center for Biotechnology Information. “PubChem Compound Summary for CID 46188928, Larotrectinib.”<https://pubchem.ncbi.nlm.nih.gov/compound/Larotrectinib, cited 23 July, 2025>.
Meertens M, Daniëlle de Jong L, Vries N de, Rosing H, Beijnen JH, Huitema ADR. LC-MS/MS method development and validation for novel targeted anticancer therapies adagrasib, capmatinib, ensartinib, entrectinib, larotrectinib, lorlatinib, pralsetinib, selpercatinib and sotorasib. Journal of Pharmaceutical and Biomedical Analysis, 117078 (2025) https://doi.org/10.1016/j.jpba.2025.117078 .
Cheruku S, Darna B, Medipalli V, Nekkalapudi AR, Sadasivam RK. Bioanalytical method development and validation for the quantitation of larotrectinib in human plasma: Application to pharmacokinetics in healthy rabbits. J Appl Pharm Sci, (2023) https://doi.org/10.7324/japs.2023.127799.
Kavitapu D, Maruthapillai A, Tamilselvi M. Identification and characterization of unknown degradation product of larotrectinib sulphate. Materials Today: Proceedings, 40, S172–9 (2021) https://doi.org/10.1016/j.matpr.2020.06.526
Fatima M, Koneru A, Ali Khan MM, Balaram Varanasi M, Pasha Syed I. Development and Validation of Stability Indicating RP-HPLC Method for Estimation of Larotrectinib in its Formulations. Orient. J. Chem, 36, 327–33 (2020) https://doi.org/10.13005/ojc/360216.
Xuan DT, Nguyen HMT, Hoang VD. Recent applications of analytical quality-by-design methodology for chromatographic analysis: A review. Chemometrics and Intelligent Laboratory Systems, 254, 105243 (2024) https://doi.org/10.1016/j.chemolab.2024.105243.
Tripathy HK, Nair Manju SV, Zakkula A, Bestha RM, Dittakavi S, Mullangi R. Validated HPLC Method for Quantification of a Novel Trk Inhibitor, Larotrectinib in Mice Plasma: Application to a Pharmacokinetic Study. Drug Res (Stuttg), 70, 101–6 (2020) https://doi.org/10.1055/a-1071-0849.
Boddala CSR, Reddy KS, Chennuru LN, Talluri MVNK. Stereo‐Selective Separation and Determination of Larotrectinib and Its Isomeric Impurities on a Novel Immobilized Cellulose Tris‐(3‐Chloro‐5‐Methyl Phenyl Carbamate) Chiral Stationary Phase by Using Normal Phase Liquid Chromatography. Separation Science Plus, 8, (2025) https://doi.org/10.1002/sscp.70089.
Bhangare D, Rajput N, Jadav T, Sahu AK, Tekade RK, Sengupta P. Systematic strategies for degradation kinetic study of pharmaceuticals: an issue of utmost importance concerning current stability analysis practices. J Anal Sci Technol, 13, (2022) https://doi.org/10.1186/s40543-022-00317-6 .
Jesus do Nascimento Lopes I de, Kazumasa Fujimori S, de Carvalho Mendes T, Adrielle Dias de Almeida R, Furtado de Mendonça de Sousa F, Areias de Oliveira C, Dibo do Nascimento D, Rebello Lourenço F, Isabel Rodrigues M, Deris Prado L. Application of analytical quality by design (AQbD) for stability-indicating method development for quantification of nevirapine and its degradation products. Microchemical Journal, 199, 109939 (2024) https://doi.org/10.1016/j.microc.2024.109939.
International Council for Harmonisation. “ICH Q14 Guideline: Analytical Procedure Development.” : <https://database.ich.org/sites/default/files/ICH_Q14_Document_Step2_Guideline_2022_0324.pdf, cited 23 July, 2025>.
International Council for Harmonisation. “ICH Q9 Guideline: Quality Risk Management.” : <https://database.ich.org/sites/default/files/Q9_Guideline.pdf, cited 23 July, 2025>.
Aggarapu S, Galla R, Shaik NJ, Dasari SJ. Application of Plackett–Burman and Box–Behnken designs to develop a sensitive and robust HPLC method for quantifying hypolipidemic drugs, rosuvastatin and bempedoic acid in tablets. Futur J Pharm Sci, 11, (2025) https://doi.org/10.1186/s43094-025-00814-6.
Salva C, Galla R. The Novel Quality by Design Concept in the Development and Validation of a Stability-Indicating RP-HPLC PDA Method for Estimating Terlipressin in an Injectable Dosage Form. Chromatographia, 87, 567–79 (2024) https://doi.org/10.1007/s10337-024-04352-w .
Narikimalli A, Galla R. AQbD based approach for UPLC procedure development for the concurrent quantification of Metformin, Vildagliptin, Dapagliflozin and Sitagliptin in bulk and tablets: Response surface methodology paradigm. Analytical Chemistry Letters, 14, 528–48 (2024) https://doi.org/10.1080/22297928.2024.2376118.
Salwa, Kumar L. Quality-by-design driven analytical method (AQbD) development and validation of HPLC–UV technique to quantify rivastigmine hydrogen tartrate in lipidic nanocarriers: Forced degradation, and assessment of drug content and in vitro release studies. Microchemical Journal, 193, 108944 (2023) https://doi.org/10.1016/j.microc.2023.108944.
International Council for Harmonisation. “ICH Harmonised Guideline: Validation of Analytical Procedures Q2(R2).” : <https://database.ich.org/sites/default/files/ICH_Q2%28R2%29_Document_Step2_Guideline_2022_0324.pdf, cited 7 June, 2025>.
U.S. Food and Drug Administration, Center for Drug Evaluation and Research. “Reviewer Guidance: Validation of Chromatographic Methods.” : <https://www.fda.gov/files/drugs/published/Analytical-Procedures-and-Methods-Validation-for-Drugs-and-Biologics.pdf, cited 9 June, 2025>.
International Council for Harmonisation. “Q1A(R2): Stability Testing of New Drug Substances and Drug Products.” : <https://database.ich.org/sites/default/files/Q1A%28R2%29%20Guideline.pdf, cited 2 June, 2025>.
Wagdy HA. A newly developed and validated environmentally friendly RP-HPLC stability indicating method for the COVID-19 antiviral Molnupiravir: Application to degradation kinetics structure suggestion using LC-MS and the effect on viable cells of the major degradation products. Microchemical Journal, 199, 109980 (2024) https://doi.org/10.1016/j.microc.2024.109980 .
Mandour AA, Nabil N, Zaazaa HE, Abdelkawy M. Review on analytical studies of some pharmaceutical compounds containing heterocyclic rings: brinzolamide, timolol maleate, flumethasone pivalate, and clioquinol. Futur J Pharm Sci, 6, (2020) https://doi.org/10.1186/s43094-020-00068-4.
Universal HPLC Solutions. “C18 HPLC Column.” 〈https://uhplcs.com/c18-hplc-column/, cited 28 July, 2025.
Kumar B, Akhtar MJ, Paul J, Singh K, Pannu S, Pal R, Khan SA. An Update on Recently Developed Analytical and Bio-analytical Methods for Some Anticancer Drugs. CPA, 19, 117–35 (2023) https://doi.org/10.2174/1573412919666221123110420.
Nuli MV, Seemaladinne R, Tallam AK. Analytical quality by design (AQbD) based optimization of RP-UPLC method for determination of nivolumab and relatlimab in bulk and pharmaceutical dosage forms. Futur J Pharm Sci, 10, (2024) https://doi.org/10.1186/s43094-024-00659-5.
Amer MM, Habib AA, Hammad SF, Kamal AH. Green micellar stability‐indicating high‐performance liquid chromatography method for determination of rupatadine fumarate in the presence of its main impurity desloratadine: Oxidative degradation kinetics study. J of Separation Science, 46, (2023) https://doi.org/10.1002/jssc.202300135.
Amer MM, Kamal AH, Hammad SF, Habib AA. Stability indicating RP‐HPLC method for methylcobalamin determination in different dosage forms: Application to photodegradation kinetics and pH rate profiling. J of Separation Science, 45, 2877–86 (2022) https://doi.org/10.1002/jssc.202200132.
Prajapati PB, Bagul N, Kalyankar G. Implementation of DoE and Risk-Based Enhanced Analytical Quality by Design Approach to Stability-Indicating RP-HPLC Method for Stability Study of Bosutinib. Journal of AOAC INTERNATIONAL, 104, 1742–53 (2021) https://doi.org/10.1093/jaoacint/qsab078.
Yin L, Yu L, Guo Y, Wang C, Ge Y, Zheng X, Zhang N, You J, Zhang Y, Shi M. Green analytical chemistry metrics for evaluating the greenness of analytical procedures. Journal of Pharmaceutical Analysis, 14, 101013 (2024) https://doi.org/10.1016/j.jpha.2024.101013.
Pena-Pereira F, Wojnowski W, Tobiszewski M. AGREE—Analytical GREEnness Metric Approach and Software. Anal. Chem., 92, 10076–82 (2020) https://doi.org/10.1021/acs.analchem.0c01887.
Darade R, Pekamwar S. Quality by design driven RP-HPLC method optimization for analysis of levothyroxine and liothyronine in bulk and tablet dosage form. J. Appl. Pharm. Res., 13, 139–48 (2025) https://doi.org/10.69857/joapr.v13i2.867.
Fitch BN, Gray R, Beres M, Hicks MB, Farrell W, Aurigemma C, Olesik SV. Life cycle analysis and sustainability comparison of reversed phase high performance liquid chromatography and carbon dioxide-containing chromatography of small molecule pharmaceuticals. Green Chem., 24, 4516–32 (2022) https://doi.org/10.1039/d1gc03750a.
Tomikj M, Božinovska M, Anevska-Stojanovska N, Lazova J, Acevska J, Brezovska K, Tonich-Ribarska J, Nakov N. Sustainable and white HPLC method for simultaneous determination of amlodipine and atorvastatin in film-coated tablet. Green Analytical Chemistry, 8, 100103 (2024) https://doi.org/10.1016/j.greeac.2024.100103.
Mansour FR, Omer KM, Płotka-Wasylka J. A total scoring system and software for complex modified GAPI (ComplexMoGAPI) application in the assessment of method greenness. Green Analytical Chemistry, 10, 100126 (2024) https://doi.org/10.1016/j.greeac.2024.100126.
Vanga MG, Bukke SPN, Kusuma PK, Narapureddy BR, Thalluri C. Integrating green analytical chemistry and analytical quality by design: an innovative approach for RP-UPLC method development of ensifentrine in bulk and inhalation formulations. BMC Chemistry, 19, (2025) https://doi.org/10.1186/s13065-025-01448-8.
Manousi N, Wojnowski W, Płotka-Wasylka J, Samanidou V. Blue applicability grade index (BAGI) and software: a new tool for the evaluation of method practicality. Green Chem., 25, 7598–604 (2023) https://doi.org/10.1039/d3gc02347h.
Saleh SS, Lotfy HM, Elbalkiny HT. An integrated framework to develop an efficient valid green (EVG) HPLC method for the assessment of antimicrobial pollutants with potential threats to human health in aquatic systems. Environ. Sci.: Processes Impacts, 25, 2125–38 (2023) https://doi.org/10.1039/d3em00339f.
Miriam Marques S, Shirodkar RK, Kumar L. Analytical ‘Quality-by-Design’ paradigm in development of a RP-HPLC method for the estimation of cilnidipine in nanoformulations: Forced degradation studies and mathematical modelling of in-vitro release studies. Microchemical Journal, 193, 109124 (2023) https://doi.org/10.1016/j.microc.2023.109124.
Prajapati P, Salunkhe M, Pulusu V, Shah S. Implementation of white analytical chemistry-driven analytical quality risk assessment and design of experiments to multipurpose chromatographic method for the synchronous estimation of multiple drugs co-formulated with paracetamol. JPC-J Planar Chromat, 37, 69–86 (2023) https://doi.org/10.1007/s00764-023-00262-z.
Nowak PM, Wietecha-Posłuszny R, Pawliszyn J. White Analytical Chemistry: An approach to reconcile the principles of Green Analytical Chemistry and functionality. TrAC Trends in Analytical Chemistry, 138, 116223 (2021) https://doi.org/10.1016/j.trac.2021.116223.
Published
How to Cite
Issue
Section
Copyright (c) 2025 Syamala P. N. S, Sreedevi Adikay

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
















