A review on the structure, distribution, and biological activities of biflavonoids in clusiaceae
DOI:
https://doi.org/10.69857/joapr.v13i5.1408Keywords:
Clusiaceae, Biflavonoids, Structural Diversity, Bioactivities, Structural Activity RelationshipAbstract
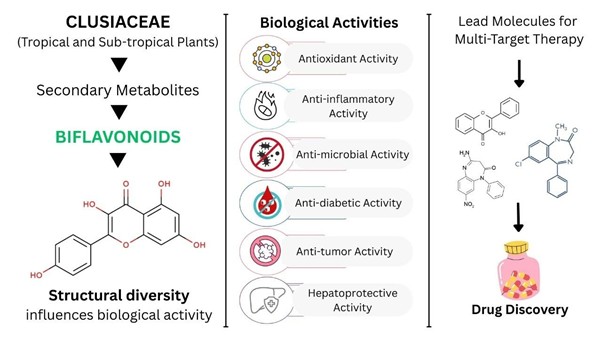
Background: The Clusiaceae family is widely distributed in tropical and subtropical climates, with a rich source of structurally diverse metabolites. Among these, biflavonoids stand out due to their complex structures and significant pharmacological activities. This review aims to examine data on various Clusiacean biflavonoids, their structural diversity, and bioactivity, thereby increasing the understanding of their implications in both traditional and modern medicine. Methodology: Data were collected from electronic databases like PubMed, Elsevier, Springer, and Google Scholar. The structural categorization of biflavonoids was based on the nature of linkage and substituent variations that influence their biological activities. Their distribution across Clusiacean members further highlights their chemotaxonomic importance. Additionally, structure-activity relationship studies reveal that specific linkages and functional groups enhance biological activity. Results and Discussion: Studies indicate that Clusiacean biflavonoids are potential candidates for drug discovery and therapeutic development due to their varied pharmacological activities. Approximately 21 biflavonoids from Clusiacean members are included in this review; nine of them exhibit antioxidant, five antimicrobial, five early antigen inhibition, four monoamine oxidase inhibition, three anti-inflammatory, three neuromuscular transmission inhibition, one antidiabetic, one anti-aging, and one hypocholesterolemic activity. Conclusion: Clusiacean biflavonoids possess desirable compounds for drug development owing to their diverse pharmacological activities, including antiviral, anti-inflammatory, anti-cancer, antioxidant, and neuroprotective properties, and so on. Overall, this article analyzes different biflavonoids obtained from Clusiaceae species, focusing on their structural diversity, biological importance, and structure-activity relationships.
Downloads
References
Kshirsagar P, Gaikwad S, Pai S, Desai N, Bapat V. Evaluation of antioxidant capacity and phytochemical investigation of eleven Clusiaceae members from Western Ghats, India. Biocatalysis and Agricultural Biotechnology, 44, 102476 (2022) https://doi.org/10.1016/j.bcab.2022.102476.
Diel KAP, Marinho LC, Von Poser GL. The ethnobotanical relevance of the tribe Symphonieae (Clusiaceae) around the world. Journal of Ethnopharmacology, 284, 114745 (2022) https://doi.org/10.1016/j.jep.2021.114745.
Raj SP, Solomon PR, Thangaraj B. Biodiesel from flowering plants. Springer, (2022) https://doi.org/10.1007/978-981-16-4775-8.
He X, Yang F, Huang X. Proceedings of Chemistry, Pharmacology, Pharmacokinetics and Synthesis of Biflavonoids. Molecules, 26, 6088 (2021) https://doi.org/10.3390/molecules26196088.
Li H, Yang J, Wang M, Ma X, Peng X. Studies on the inhibition of α-glucosidase by biflavonoids and their interaction mechanisms. Food Chemistry, 420, 136113 (2023) https://doi.org/10.1016/j.foodchem.2023.136113.
Klischan MKT, Mazzone F, Berning L, Greb J, Schlamkow M, Haase M, Frey W, Stork B, Pfeffer K, Pietruszka J. Modular Approach for the Synthesis and Bioactivity Profiling of 8,8′-Biflavones. ACS Omega, 8, 41816-34 (2023) https://doi.org/10.1021/acsomega.3c06503.
Šamec D, Jurčević Šangut I, Karalija E, Šarkanj B, Zelić B, Šalić A. 3′-8″- Biflavones: A Review of Their Structural Diversity, Natural Occurrence, Role in Plants, Extraction and Identification. Molecules, 29, 4634 (2024) https://doi.org/10.3390/molecules29194634.
Goossens J-F, Goossens L, Bailly C. Hinokiflavone and Related C–O–C-Type Biflavonoids as Anti-cancer Compounds: Properties and Mechanism of Action. Nat. Prod. Bioprospect., 11, 365-77 (2021) https://doi.org/10.1007/s13659-021-00298-w.
Wu S, Liu C, Li Y, Zhang X, Han Q, Zhao H, Zhao K, Dang Y, Wang R, Song S. Inhibitory mechanisms of Amentoflavone on amyloid-β peptide aggregation revealed by replica exchange molecular dynamics. Sci Rep, 15, 24352 (2025) https://doi.org/10.1038/s41598-025-10623-9.
Carrillo-Hormaza L, Ramírez AM, Osorio E. Chemometric classification of Garcinia madruno raw material: Impact of the regional origin and ripeness stage of a Neotropical exotic species. Food Chemistry, 293, 291–8 (2019) https://doi.org/10.1016/j.foodchem.2019.04.118.
Chen H-X, Yang F, He X-Q, Li T, Sun Y-Z, Song J-P, Huang X-A, Guo W-F. Garcinia Biflavonoid 1 Improves Lipid Metabolism in HepG2 Cells via Regulating PPARα. Molecules, 27, 1978 (2022) https://doi.org/10.3390/molecules27061978.
Hioki Y, Onwona-Agyeman S, Kakumu Y, Hattori H, Yamauchi K, Mitsunaga T. Garcinoic Acids and a Benzophenone Derivative from the Seeds of Garcinia kola and Their Antibacterial Activities against Oral Bacterial Pathogenic Organisms. J. Nat. Prod., 83, 2087–92 (2020) https://doi.org/10.1021/acs.jnatprod.9b01045.
Fu T-D, Zhang W-L, Zhang Y, Deng C-S, Qi W, Xu Q, Song J-P, Guo W-F, Yang R-Y, Huang X-A. Garcinia Biflavonoid 1 from Garcinia kola Ameliorates Glycolipid Metabolism Disorder in Type 2 Diabetic db/db Mice. Natural Product Communications, 19, 1934578X231224993 (2024) https://doi.org/10.1177/1934578X231224993.
Alrazi IMD, Ogunwa TH, Kolawole AO, Elekofehinti OO, Omotuyi OI, Miyanishi T, Maruta S. Kolaflavanone, a biflavonoid derived from medicinal plant Garcinia, is an inhibitor of mitotic kinesin Eg5. The Journal of Biochemistry, 170, 611–22 (2021) https://doi.org/10.1093/jb/mvab083
Kshirsagar P, Gaikwad S, Pai S, Desai N, Bapat V. Evaluation of antioxidant capacity and phytochemical investigation of eleven Clusiaceae members from Western Ghats, India. Biocatalysis and Agricultural Biotechnology, 44, 102476 (2022) https://doi.org/10.1016/j.bcab.2022.102476.
Dai X-H, Zhu J-M, Wang G-Y, Ren Y-H, Liu H-L,Wang Y. Gymnosperm-specific CYP90Js enable biflavonoid biosynthesis and microbial production of Amentoflavone. Nat Commun, 16, 7792 (2025) https://doi.org/10.1038/s41467-025-62990-6.
Ferraz CG, Silva MDCC, Pereira DASG, Caldas BVV, Mattos R, Oliveira VVG, Andrade EMJ, Soares ACF, Da Silva F, Cruz FG, Ribeiro PR. Polyprenylated benzophenone derivatives from Clusia burle-marxii and their chemotaxonomic significance. Biochemical Systematics and Ecology, 94, 104218 (2021) https://doi.org/10.1016/j.bse.2020.104218.
Téné D-G, Tih AE, Kamdem MHK, Talla RM, Diboue PHB, Melongo YKD, Dzukoug CR, Mmutlane EM, Ndinteh DT, Bodo B, Ghogomu RT. Antibacterial and antioxidant activities of compounds isolated from the leaves of Symphonia globulifera (Clusiaceae) and their chemophenetic significance. Biochemical Systematics and Ecology, 99, 104345 (2021) https://doi.org/10.1016/j.bse.2021.104345.
Ferdosh S. The Extraction of Bioactive Agents from Calophyllum inophyllum L., and Their Pharmacological Properties. Sci. Pharm., 92, 6 (2024) https://doi.org/10.3390/scipharm92010006.
Rizzo P, Altschmied L, Ravindran BM, Rutten T, D’Auria JC. The Biochemical and Genetic Basis for the Biosynthesis of Bioactive Compounds in Hypericum perforatum L., One of the Largest Medicinal Crops in Europe. Genes, 11, 1210 (2020) https://doi.org/10.3390/genes11101210.
Phukhatmuen P, Raksat A, Laphookhieo S, Charoensup R, Duangyod T, Maneerat W. Bioassay guided isolation and identification of antidiabetic compounds from Garcinia cowa leaf extract. Heliyon, 6, e03625 (2020) https://doi.org/10.1016/j.heliyon.2020.e03625.
Siangcham T., Prathaphan P., Ruangtong J., Thongsepee N., Martviset P., Chantree P., Sornchuer P., and Sangpairoj K., Camboginol and Morelloflavone from Garcinia dulcis (Roxb.) Kurz flower extract promotes autophagic cell death against human glioblastoma cells through endoplasmic reticulum stress. Prev Nutr Food Sci., 27, 376-383, (2022) https://doi.org/10.3746/pnf.2022.27.4.376.
Tuansulong K, Hutadilok‐Towatana N, Mahabusarakam W, Pinkaew D, Fujise K. Morelloflavone from Garcinia dulcis as a Novel Biflavonoid Inhibitor of HMG‐CoA Reductase. Phytotherapy Research, 25, 424–8 (2011) https://doi.org/10.1002/ptr.3286.
Magadula J, Mbwambo Z, Gatto J, Derbré S, Guilet D, Richomme P. Polyphenolic Compounds with Anti-Ages Activity from Three Clusiaceae Plants. EJMP, 4, 1336–44 (2014) https://doi.org/10.9734/EJMP/2014/10812.
Recalde-Gil MA, Klein-Júnior LC, Dos Santos Passos C, Salton J, De Loreto Bordignon SA, Monace FD, Filho VC, Henriques AT. Monoamine Oxidase Inhibitory Activity of Biflavonoids from Branches of Garcinia gardneriana (Clusiaceae). Natural Product Communications, 12, 1934578X1701200411 (2017) https://doi.org/10.1177/1934578X1701200411.
Othman NA, Liew SY, Khaw KY, Ablat A, Karsani SA, Leong KH, Blanchard P, Derbré S, Awang K. Secondary metabolites from the barks of Garcinia griffithii T. Anderson (Clusiaceae) and their chemophenetic significance. Biochemical Systematics and Ecology, 116, 104869 (2024) https://doi.org/10.1016/j.bse.2024.104869.
Sazali NSAN, Ahmad F, Taher M, Salleh WMNHW. Biflavonoids from the leaves and stem bark of Garcinia griffithii and their biological activities. mpj, 21, 889–97 (2017) https://doi.org/10.12991/mpj.2017.27.
Ito C, Itoigawa M, Miyamoto Y, Rao KS, Takayasu J, Okuda Y, Mukainaka T, Tokuda H, Nishino H, Furukawa H. A New Biflavonoid from Calophyllum panciflorum with Antitumor-Promoting Activity. J. Nat. Prod., 62, 1668–71 (1999) https://doi.org/10.1021/np990065j.
Gontijo VS, De Souza TC, Rosa IA, Soares MG, Da Silva MA, Vilegas W, Viegas C, Dos Santos MH. Isolation and evaluation of the antioxidant activity of phenolic constituents of the Garcinia brasiliensis epicarp. Food Chemistry, 132, 1230–5 (2012) https://doi.org/10.1016/j.foodchem.2011.10.110.
Saroni Arwa P, Zeraik ML, Farias Ximenes V, Da Fonseca LM, Da Silva Bolzani V, Siqueira Silva DH. Redox-active biflavonoids from Garcinia brasiliensis as inhibitors of neutrophil oxidative burst and human erythrocyte membrane damage. Journal of Ethnopharmacology, 174, 410–8 (2015) https://doi.org/10.1016/j.jep.2015.08.041.
Cane HPCA, Saidi N, Yahya M, Darusman D, Erlidawati E, Safrida S, Musman M. Macrophylloflavone: A New Biflavonoid from Garcinia macrophylla Mart. (Clusiaceae) for Antibacterial, Antioxidant, and Anti-Type 2 Diabetes Mellitus Activities. The Scientific World Journal, 2020, 1-14 (2020) https://doi.org/10.1155/2020/2983129.
Djoufack GLN, Valant-Vetschera KM, Schinnerl J, Brecker L, Lorbeer E, Robien W. Xanthones, Biflavanones and Triterpenes from Pentadesma grandifolia (Clusiaceae): Structural Determination and Bioactivity. Natural Product Communications, 5, 1934578X1000500714 (2010) https://doi.org/10.1177/1934578X1000500714.
Rufa’i, F. A., Baecker, D., & Mukhtar, M. D. Phytochemical Screening, GC-MS Analysis, and Evaluating In Vivo Antitrypanosomal Effects of a Methanolic Extract of Garcinia kola Nuts on Rats. Antibiotics, 12, 713 (2023) https://doi.org/10.3390/antibiotics12040713
Xu H-X, Mughal S, Taiwo O, Lee SF. Isolation and characterization of an antibacterial biflavonoid from an African chewing stick Garcinia kola Heckel (Clusiaceae). Journal of Ethnopharmacology, 147, 497–502 (2013) https://doi.org/10.1016/j.jep.2013.03.047.
Patterson S, Waters ME, Braman N, Willson R, Hill RA, Magolan J, Hofmann T, Stark TD, Balemba OB. Garcinia buchananii stem bark extract and its bioactive constituents Manniflavanone, GB-2 and Buchananiflavonone attenuate intestinal inhibitory neuromuscular transmission. J Smooth Muscle Res, 59, 34-57 (2023) https://doi.org/10.1540/jsmr.59.34.
Olatoye FJ, Akindele AJ. Ninety-day oral toxicological profiling of Kolaviron (an extract of Garcinia kola) in male and female rats. Drug and Chemical Toxicology, 46, 1–14 (2023) https://doi.org/10.1080/01480545.2021.1997543.
Abdullah I, Phongpaichit S, Voravuthikunchai SP, Mahabusarakam W. Prenylated biflavonoids from the green branches of Garcinia dulcis. Phytochemistry Letters, 23, 176–9 (2018) https://doi.org/10.1016/j.phytol.2017.12.004.
Shamsudin NF, Ahmed QU, Mahmood S, Ali Shah SA, Khatib A, Mukhtar S, Alsharif MA, Parveen H, Zakaria ZA. Antibacterial Effects of Flavonoids and Their Structure-Activity Relationship Study: A Comparative Interpretation. Molecules, 27, 1149 (2022) https://doi.org/10.3390/molecules27041149.
Chen J, He N, Wang Q, Wu G, Wu W, Xin Q, Cheng G, Sang Z, Zhu C, Wu Y, Wei R, Ma Q. A Comprehensive Review of Structures, Structure-activity Relationships, Extractions, and Bioactivities of Flavonoids from Citrus medica. CCHTS, 26, 2411-23 (2023) https://doi.org/10.2174/1386207326666230330083136.
Kumar A. Amentoflavone, a biflavonoid with potential anticancer properties against viral oncoprotein E6 in Human papilloma virus-16 (HPV-16) positive cervical cancer cells. Natural Product Research, 1–10 (2024) https://doi.org/10.1080/14786419.2024.2438273.
Li X, Wang L, Han W, Mai W, Han L, Chen D. Amentoflavone Protects against Hydroxyl Radical-induced DNA Damage via Antioxidant Mechanism. Turk J Bioch, 39, 30–6 (2014) https://doi.org/10.5505/tjb.2014.65882.
Zhang G, Jing Y, Zhang H, Ma E, Guan J, Xue F, Liu H, Sun X. Isolation and Cytotoxic Activity of Selaginellin Derivatives and Biflavonoids from Selaginella tamariscina. Planta Med, 78, 390–2 (2012) https://doi.org/10.1055/s-0031-1298175.
Ayepola OR, Brooks NL, Oguntibeju OO. Kolaviron Improved Resistance to Oxidative Stress and Inflammation in the Blood (Erythrocyte, Serum, and Plasma) of Streptozotocin-Induced Diabetic Rats. The Scientific World Journal, 2014, 1–8 (2014) https://doi.org/10.1155/2014/921080.
Li X, Jiang Q, Wang T, Liu J, Chen D. Comparison of the Antioxidant Effects of Quercitrin and Isoquercitrin: Understanding the Role of the 6″-OH Group. Molecules, 21, 1246 (2016) https://doi.org/10.3390/molecules21091246.
Anaya‐Eugenio GD, Blanco Carcache PJ, Ninh TN, Ren Y, Soejarto DD, Kinghorn AD. A pentamethoxylated flavone from Glycosmis ovoidea promotes apoptosis through the intrinsic pathway and inhibits migration of MCF ‐7 breast cancer cells. Phytotherapy Research, 35, 1634–45 (2021) https://doi.org/10.1002/ptr.6930.
Wätjen W, Weber N, Lou Y -j., Wang Z -q., Chovolou Y, Kampkötter A, Kahl R, Proksch P. Prenylation enhances cytotoxicity of apigenin and liquiritigenin in rat H4IIE hepatoma and C6 glioma cells. Food and Chemical Toxicology, 45, 119–24 (2007) https://doi.org/10.1016/j.fct.2006.08.008.
Chen B, Wang X, Zhang Y, Huang K, Liu H, Xu D, Li S, Liu Q, Huang J, Yao H, Lin X. Improved solubility, dissolution rate, and oral bioavailability of main biflavonoids from Selaginella doederleinii extract by amorphous solid dispersion. Drug Delivery, 27, 309-22 (2020) https://doi.org/10.1080/10717544.2020.1716876.
Liao S, Ren Q, Yang C, Zhang T, Li J, Wang X, Qu X, Zhang X, Zhou Z, Zhang Z, Wang S. Liquid Chromatography–Tandem Mass Spectrometry Determination and Pharmacokinetic Analysis of Amentoflavone and Its Conjugated Metabolites in Rats. J. Agric. Food Chem., 63, 1957–66 (2015) https://doi.org/10.1021/jf5019615.
Feng X, Chen Y, Li L, Zhang Y, Zhang L, Zhang Z. Preparation, evaluation and metabolites study in rats of novel Amentoflavone-loaded TPGS/Soluplus mixed Nanomicelles. Drug Delivery, 27, 137–50 (2020) https://doi.org/10.1080/10717544.2019.1709920.
Ghosh A, Banerjee T, Surolia A, Bhandary S. Formulation of nanotized curcumin and demonstration of its antimalarial efficacy. IJN, 5373 (2014) https://doi.org/10.2147/IJN.S62756.
Zhao F, Qian Y, Li H, Yang Y, Wang J, Yu W, Li M, Cheng W, Shan L. Amentoflavone-loaded nanoparticles enhanced chemotherapy efficacy by inhibition of AKR1B10. Nanotechnology, 33, 385101 (2022) https://doi.org/10.1088/1361-6528/ac7810.
Yuan D, Guo Y, Pu F, Yang C, Xiao X, Du H, He J, Lu S. Opportunities and challenges in enhancing the bioavailability and bioactivity of dietary flavonoids: A novel delivery system perspective. Food Chemistry, 430, 137115 (2024) https://doi.org/10.1016/j.foodchem.2023.137115.
Published
How to Cite
Issue
Section
Copyright (c) 2025 Anjana Unni, Sheeja T Tharakan

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
















