Investigation of potential efficacy of nanospanlastic vesicular drug delivery system for targeting the brain: formulation, characterization, and in-vivo studies
DOI:
https://doi.org/10.69857/joapr.v13i4.1340Keywords:
Edaravone, nanospanlastics, nasal formulation, brain targeting, quality by designAbstract
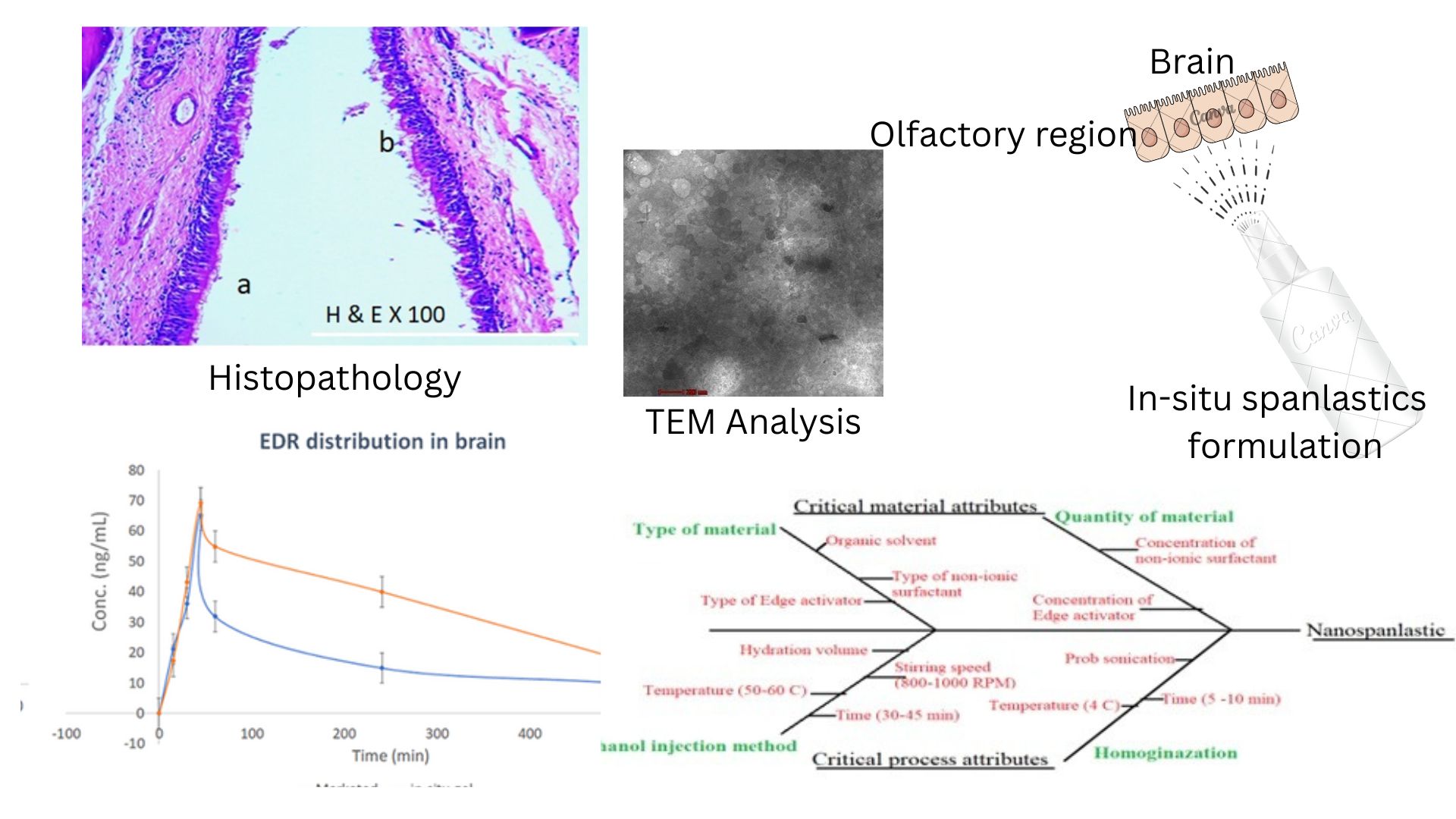
Background: Edaravone, a potent antioxidant, has limited brain bioavailability due to poor solubility and restricted permeability across the blood-brain barrier (BBB). Intranasal delivery offers a promising alternative for brain targeting by bypassing the BBB. Objective: To develop and evaluate a nanospanlastic-based in-situ nasal gel formulation of edaravone for enhanced brain delivery. Methodology: A Quality by Design (QbD) approach was employed to identify and optimize critical formulation variables using Plackett-Burman and Central Composite Design. The optimized nanospanlastics were incorporated into a gellan gum-based ion-activated in-situ nasal gel and characterized through in vitro, ex vivo, and in vivo studies. Results and Discussion: The optimized formulation exhibited a particle size of 213.4 nm, a drug entrapment efficiency of 67.59%, and rapid gelation upon contact with nasal fluid. In vitro diffusion showed over 80% drug release within 30 minutes, while ex vivo studies confirmed improved permeation (flux: 7.8067 µg/cm²/hr). Histopathology revealed no nasal mucosal irritation. Pharmacokinetic studies in rats demonstrated significantly enhanced brain and plasma exposure compared to the marketed edaravone injection, with higher Cmax (78.73 ng/mL), Tmax (121.2 min), and AUC. Conclusion: The developed nanospanlastic-based nasal gel offers a non-invasive, effective strategy for brain delivery of edaravone, with potential to improve therapeutic outcomes in neurological disorders.
Downloads
References
Ghosh A, Majie A, Karmakar V, Chatterjee K, Chakraborty S, Pandey M, Jain N, Roy Sarkar S, Nair AB, Gorain B. In-depth Mechanism, Challenges, and Opportunities of Delivering Therapeutics in Brain Using Intranasal Route. AAPS PharmSciTech, 25, 96 (2024) https://doi.org/10.1208/s12249-024-02810-0.
Yamashita T, Abe K. Update on Antioxidant Therapy with Edaravone: Expanding Applications in Neurodegenerative Diseases. Int J Mol Sci, 25, 2945 (2024) https://doi.org/10.3390/ijms25052945.
Koo J, Lim C, Oh KT. Recent Advances in Intranasal Administration for Brain-Targeting Delivery: A Comprehensive Review of Lipid-Based Nanoparticles and Stimuli-Responsive Gel Formulations. Int J Nanomedicine, 19, 1767–807 (2024) https://doi.org/10.2147/IJN.S439181.
Sharma, S. Pahwa, S. Bhati, P. Kudeshia. Spanlastics: A modern approach for nanovesicular drug delivery system. Int J Pharm Sci Res, 11, 1057–65 (2020) http://dx.doi.org/10.13040/IJPSR.0975-8232.11(3).1057-65
Ge X, Wei M, He S, Yuan W-E. Advances of Non-Ionic Surfactant Vesicles (Niosomes) and Their Application in Drug Delivery. Pharmaceutics, 11, 55 (2019) https://doi.org/10.3390/pharmaceutics11020055.
Abdelmonem R, el Nabarawi M, Attia A. Development of novel bioadhesive granisetron hydrochloride spanlastic gel and insert for brain targeting and study their effects on rats. Drug Deliv, 25, 70–7 (2018) https://doi.org/10.1080/10717544.2017.1413447.
Giri S, Markandeywar TS, Irfan Z, Manna S. Gellan gum and sodium alginate in-situ gel of monocaprin for effective corneal permeation. Food Hydrocolloids for Health, 4, 100156 (2023) https://doi.org/10.1016/j.fhfh.2023.100156.
Trivedi R, Minglani VV, El-Gazzar AM, Batiha GE-S, Mahmoud MH, Patel M, Patel M. Optimization of Pramipexole-Loaded In Situ Thermosensitive Intranasal Gel for Parkinson’s Disease. Pharmaceuticals, 17, 172 (2024) https://doi.org/10.3390/ph17020172
Shah P, Goodyear B, Haq A, Puri V, Michniak-Kohn B. Evaluations of Quality by Design (QbD) Elements Impact for Developing Niosomes as a Promising Topical Drug Delivery Platform. Pharmaceutics, 12, 246-262 (2020) https://doi.org/10.3390/pharmaceutics12030246.
Mkam Tsengam IK, Omarova M, Kelley EG, McCormick A, Bothun GD, Raghavan SR, John VT. Transformation of Lipid Vesicles into Micelles by Adding Nonionic Surfactants: Elucidating the Structural Pathway and the Intermediate Structures. J Phys Chem B, 126, 2208–16 (2022) https://doi.org/10.1021/acs.jpcb.1c09685.
Gadhave D, Rasal N, Sonawane R, Sekar M, Kokare C. Nose-to-brain delivery of teriflunomide-loaded lipid-based carbopol-gellan gum nanogel for glioma: Pharmacological and in vitro cytotoxicity studies. Int J Biol Macromol, 167, 906–20 (2021) https://doi.org/10.1016/j.ijbiomac.2020.11.047.
Jelkmann M, Leichner C, Zaichik S, Laffleur F, Bernkop-Schnürch A. A gellan gum derivative as in-situ gelling cationic polymer for nasal drug delivery. Int J Biol Macromol, 158, 1037–46 (2020) https://doi.org/10.1016/j.ijbiomac.2020.04.114.
Salatin S, Barar J, Barzegar-Jalali M, Adibkia K, Milani MA, Jelvehgari M. Hydrogel nanoparticles and nanocomposites for nasal drug/vaccine delivery. Arch Pharm Res, 39, 1181–92 (2016) https://doi.org/10.1007/s12272-016-0782-0.
Mardikasari SA, Katona G, Budai-Szűcs M, Sipos B, Orosz L, Burián K, Rovó L, Csóka I. Quality by design-based optimization of in situ ionic-sensitive gels of amoxicillin-loaded bovine serum albumin nanoparticles for enhanced local nasal delivery. Int J Pharm, 645, 123435 (2023) https://doi.org/10.1016/j.ijpharm.2023.123435.
Guillot AJ, Martínez-Navarrete M, Garrigues TM, Melero A. Skin drug delivery using lipid vesicles: A starting guideline for their development. Journal of Controlled Release, 355, 624–54 (2023) https://doi.org/10.1016/j.jconrel.2023.02.006.
Kim JK, Zeb A, Qureshi OS, Kim H-S, Cha J-H, Kim H-S. Improved skin permeation of methotrexate via nanosized ultradeformable liposomes. Int J Nanomedicine, 11, 3813–24 (2016) https://doi.org/10.2147/IJN.S109565.
Published
How to Cite
Issue
Section
Copyright (c) 2025 Ashwini Patel, Prachi Pandey

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
















