Chitosan-based mucoadhesive patches for buccal delivery of olmesartan in hypertension treatment
DOI:
https://doi.org/10.69857/joapr.v13i4.1298Keywords:
Hypertension, Buccal patches, Olmesartan-Chitosan, hypertension, mucoadhesiveAbstract
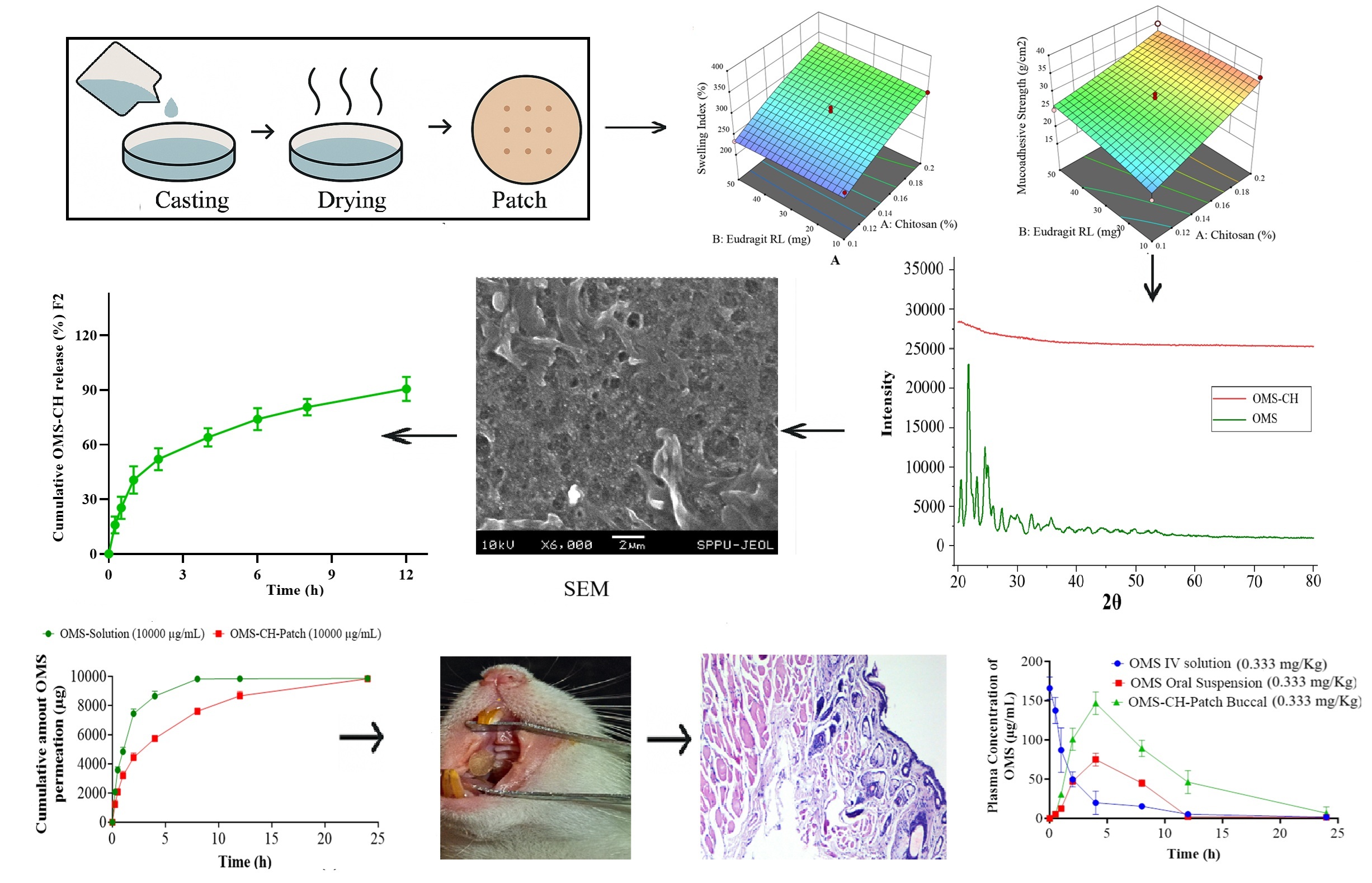
Background: Delivering poorly soluble drugs like Olmesartan (OMS) effectively remains a key challenge due to low oral bioavailability and extensive first-pass metabolism. To address this, buccal patches incorporating chitosan were developed as an alternative route to enhance systemic absorption. Methodology: A series of buccal patch formulations (F1–F17) was prepared using combinations of chitosan, polyvinyl alcohol (PVA), HPMC K4M, and Eudragit RL via solvent casting. These patches were evaluated for uniformity in weight, thickness, pH, mechanical strength, folding endurance, and mucoadhesion. Structural and morphological assessments were carried out using X-ray diffraction and SEM. Ex vivo and in vivo studies explored drug release, permeation, pharmacokinetics, and mucosal safety. An HPLC method was employed for accurate quantification, and stability was assessed under both accelerated and ambient conditions. Results and Discussion: The optimised patch (F2) demonstrated consistent physical properties, high flexibility, and strong mucoadhesion. XRD patterns confirmed the amorphous dispersion of OMS in the polymer matrix, aiding solubility. Drug release was sustained over 12 hours, and permeation studies showed controlled transport across the buccal membrane. In vivo results revealed a substantial improvement in drug bioavailability via buccal delivery (83.2%) compared to oral administration (30.2%). Histological analysis indicated no signs of tissue irritation. Patches maintained integrity and potency throughout six months of storage. Conclusion: The findings support the buccal patch as a viable, non-invasive platform for enhancing OMS delivery, offering improved therapeutic efficiency and patient compliance.
Downloads
References
Zhou B, Perel P, Mensah GA, Ezzati M. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat. Rev. Cardiol., 18, 785–802 (2021) https://doi.org/10.1038/s41569-021-00559-8
Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat. Rev. Nephrol., 16, 223–37 (2020) https://doi.org/10.1038/s41581-019-0244-2
Qelliny MR, Mustafa WW, Al Fatease A, Alamri AH, Alany R, Abdelkader H. Biofunctional Excipients: Their Emerging Role in Overcoming the Inherent Poor Biopharmaceutical Characteristics of Drugs. Pharmaceutics, 17, 598 (2025) https://doi.org/10.3390/pharmaceutics17050598
Le NN, Frater I, Lip S, Padmanabhan S. Hypertension precision medicine: the promise and pitfalls of pharmacogenomics. Pharmacogenomics, 1–24 (2025) https://doi.org/10.1080/14622416.2025.2504865
Rind L, Mahmood T, Siddiqui MH, Ahsan F, Shamim A, Anwar A, Yadav RK. From Hypertension to Beyond: Unraveling the Diverse Mechanisms of Olmesartan in Disease Modulation. Drug Res. (Stuttg)., 74, 93–101 (2024) https://doi.org/10.1055/a-2244-3136
Agata J, Ura N, Yoshida H, Shinshi Y, Sasaki H, Hyakkoku M, Taniguchi S, Shimamoto K. Olmesartan is an angiotensin II receptor blocker with an inhibitory effect on angiotensin-converting enzyme. Hypertens. Res., 29, 865–74 (2006) https://doi.org/10.1291/hypres.29.865
Chaure VA, Shirkhedkar AA. An Investigative Review for PharmaceuticalAnalysis of Angiotensin receptor blockers: Olmesartan Medoxomil, Annales Pharmaceutiques Françaises, 83, 33-44 (2024) https://doi.org/10.1016/j.pharma.2024.10.001
Lee BS, Kang MJ, Choi WS, Choi YB, Kim HS, Lee SK, Lee J, Choi YW. Solubilized formulation of olmesartan medoxomil for enhancing oral bioavailability. Arch. Pharm. Res., 32, 1629–35 (2009) https://doi.org/10.1007/s12272-009-2117-x
Tamargo J, Ruilope LM. Investigational calcium channel blockers for the treatment of hypertension. Expert Opin. Investig. Drugs, 25, 1295–309 (2016) https://doi.org/10.1080/13543784.2016.1241764
Godfraind T. Discovery and development of calcium channel blockers. Front. Pharmacol., 8, 286 (2017) https://doi.org/10.3389/fphar.2017.00286
Mathias NR, Hussain MA. Non-invasive systemic drug delivery: developability considerations for alternate routes of administration. J. Pharm. Sci., 99, 1–20 (2010) https://doi.org/10.1002/jps.21793
Junginger HE, Hoogstraate JA, Verhoef JC. Recent advances in buccal drug delivery and absorption—in vitro and in vivo studies. J. Control. release, 62, 149–59 (1999) https://doi.org/10.1016/S0168-3659(99)00032-2
Rossi S, Sandri G, Caramella CM. Buccal drug delivery: a challenge already won? Drug Discov. Today Technol., 2, 59–65 (2005) https://doi.org/10.1016/j.ddtec.2005.05.018
Patel VF, Liu F, Brown MB. Modeling the oral cavity: in vitro and in vivo evaluations of buccal drug delivery systems. J. Control. release, 161, 746–56 (2012) https://doi.org/10.1016/j.jconrel.2012.05.026
Mura P, Maestrelli F, Cirri M, Mennini N. Multiple roles of chitosan in mucosal drug delivery: an updated review. Mar. Drugs, 20, 335 (2022) https://doi.org/10.3390/md20050335
Patel VM, Prajapati BG, Patel MM. Design and characterization of chitosan-containing mucoadhesive buccal patches of propranolol hydrochloride. Acta Pharm., 57, 61–72 (2007) https://doi.org/10.2478/v10007-007-0005-9
Jadhav S, Mishra S. The spray-dried mucoadhesive microparticles of rizatriptan with chitosan and carbopol in migraine. Egypt. Pharm. J., 21, 293–301 (2022) https://dx.doi.org/10.4103/epj.epj_37_22
Jadhav SM, Mishra SK. Spray Dried Mucoadhesive Microparticles of Donepezil with Chitosan and Carbopol in Alzheimer’s Disease. Int. J. Pharm. Investig., 12, (2022) https://doi.org/10.5530/ijpi.2022.2.36
Navamanisubramanian R, Nerella R, Duraipandian C, Seetharaman S. Quality by design approach for optimization of repaglinide buccal tablets using Box-Behnken Design. Futur. J. Pharm. Sci., 4, 265–72 (2018) https://doi.org/10.1016/j.fjps.2018.10.002
Hashemi M, Ramezani V, Seyedabadi M, Ranjbar AM, Jafari H, Honarvar M, Fanaei H. Formulation and optimization of oral mucoadhesive patches of myrtus communis by box behnken design. Adv. Pharm. Bull., 7, 441 (2017) https://doi.org/10.15171/apb.2017.053
Baus RA, Haug MF, Leichner C, Jelkmann M, Bernkop-Schnürch A. In vitro–in vivo correlation of mucoadhesion studies on buccal mucosa. Mol. Pharm., 16, 2719–27 (2019) https://doi.org/10.1021/acs.molpharmaceut.9b00254
Kumar M, Sharma A, Mahmood S, Thakur A, Mirza MA, Bhatia A. Franz diffusion cell and its implication in skin permeation studies. J. Dispers. Sci. Technol., 45, 943–56 (2024) https://doi.org/10.1080/01932691.2023.2188923
Paarakh MP, Jose PA, Setty CM, Peterchristoper G V. Release kinetics–concepts and applications. Int. J. Pharm. Res. Technol., 8, 12–20 (2018) https://doi.org/10.31838/ijprt/08.01.02
Askarizadeh M, Esfandiari N, Honarvar B, Sajadian SA, Azdarpour A. Kinetic modeling to explain the release of medicine from drug delivery systems. ChemBioEng Rev., 10, 1006–49 (2023) https://doi.org/10.1002/cben.202300027
Devadhe AS, Dighe SB, Yadav SS, Bhawar SB, Ghogare RD. Antioxidant and hepatoprotective activity of Nigella sativa alcoholic extract in a CCl4-induced rat. J. Appl. Pharm. Res., 13, 115–26 (2025) https://doi.org/10.69857/joapr.v13i2.985.
Pawar O, Godge R, Shinde G, Barde K, Vikhe A. Design, Development, and Optimization of Mucoadhesive Buccal Films of Ganaxolone for Enhanced Bioavailability. J. Appl. Pharm. Res., 13, 95–107 (2025) https://doi.org/10.69857/joapr.v13i2.943.
Jaipakdee N, Pongjanyakul T, Limpongsa E. Preparation and characterization of poly (vinyl alcohol)-poly (vinyl pyrrolidone) mucoadhesive buccal patches for delivery of lidocaine HCL. Int. J. Appl. Pharm, 10, 115–23 (2018) http://dx.doi.org/10.22159/ijap.2018v10i1.23208
Aggarwal D, Gupta RD, Sharma V. Qbd Enabled Optimization Study of the Variable Concentration of Phospholipid and Stabilizer in the Development of Liposomal Pastilles of Solid Dispersion Polymeric Composite of Antihypertensive Drug. J. Appl. Pharm. Res., 13, 259–71 (2025) https://doi.org/10.69857/joapr.v13i3.995.
Pranay R, Tatikayala RK, Damera S, Pathakala N, Jadi RK. Insights of Nose To Brain Delivery in Treating Parkinson’S Disease: a Systematic Review. J. Appl. Pharm. Res., 12, 57–72 (2024) https://doi.org/10.69857/joapr.v12i6.625.
Coluzza I, van Oostrum PDJ, Capone B, Reimhult E, Dellago C. Design and folding of colloidal patchy polymers. Soft Matter, 9, 938–44 (2013) https://doi.org/10.1039/C2SM26967H
Cilurzo F, Gennari CGM, Minghetti P. Adhesive properties: a critical issue in transdermal patch development. Expert Opin. Drug Deliv., 9, 33–45 (2012) https://doi.org/10.1517/17425247.2012.637107
Nair AB, Kumria R, Harsha S, Attimarad M, Al-Dhubiab BE, Alhaider IA. In vitro techniques to evaluate buccal films. J. Control. Release, 166, 10–21 (2013) https://doi.org/10.1016/j.jconrel.2012.11.019
Rohani Shirvan A, Hemmatinejad N, Bahrami SH, Bashari A. Fabrication of multifunctional mucoadhesive buccal patch for drug delivery applications. J. Biomed. Mater. Res. Part A, 109, 2640–56 (2021) https://doi.org/10.1002/jbm.a.37257
Shelke P V, Rachh PR, Mankar S, Amin S, Jain D. Optimization and evaluation of nebivolol hydrochloride loaded transferosomes using Box-Behnken experimental design. J. Appl. Pharm. Res., 12, 124–38 (2024) https://doi.org/10.69857/joapr.v12i4.590.
Hassan MA, Barakat NS, El-Badry M, Shehata SM. Formulation and in vitro/in vivo evaluation of naproxen mucoadhesive buccal patches for local effect. J. Drug Deliv. Sci. Technol., 21, 423 (2011) https://doi.org/10.1016/S1773-2247(11)50068-1
Sagirli O, Önal A, Toker SE, Şensoy D. Simultaneous HPLC analysis of olmesartan and hydrochlorothiazide in combined tablets and in vitro dissolution studies. Chromatographia, 66, 213–8 (2007) https://doi.org/10.1365/s10337-007-0304-9
Al-Ali M, Al-Ali LI. Modeling Kinetics and Transport Mechanism Study of Poorly Soluble Drug Formulation in High Acidic Medium. Tikrit J. Eng. Sci., 31, 76–91 (2024) https://doi.org/10.25130/tjes.31.4.8
Rajab NA, Sulaiman HT. Olmesartan Medoxomil Nanomicelle Using Soluplus for Dissolution Enhancement: Preparation, In-vitro and Ex-vivo Evaluation. Iraqi J. Pharm. Sci., 34, 47–59 (2025) https://doi.org/10.31351/vol34iss2pp47-59
Landry L, Dong X. Investigation of hydrolysis of olmesartan medoxomil in different pH buffers by simultaneously measuring olmesartan medoxomil and olmesartan. PLoS One, 20, e0321142 (2025) https://doi.org/10.1371/journal.pone.0321142
Shivaraj D, Bellaiah PG, Srinivasa S. Development and characterization of Olmesartan Medoximil Self-Microemulsifying Fast Disintegrating Tablet. J. Drug Deliv. Ther., 15, (2025) https://orcid.org/0009-0006-6790-1235
Gannu R, Vamshi Vishnu Y, Kishan V, Madhusudan Rao Y. Development of nitrendipine transdermal patches: in vitro and ex vivo characterization. Curr. Drug Deliv., 4, 69–76 (2007) https://doi.org/10.2174/156720107779314767
Published
How to Cite
Issue
Section
Copyright (c) 2025 Ram Nikhate, Sanjay Patil

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
















