Development of abemaciclib-encapsulated nanosponges for breast cancer: optimization, drug release kinetics, and in vitro efficacy
DOI:
https://doi.org/10.69857/joapr.v13i4.1234Keywords:
Abemaciclib, nanoparticle, nanosponges, breast cancer, emulsion-solvent diffusionAbstract
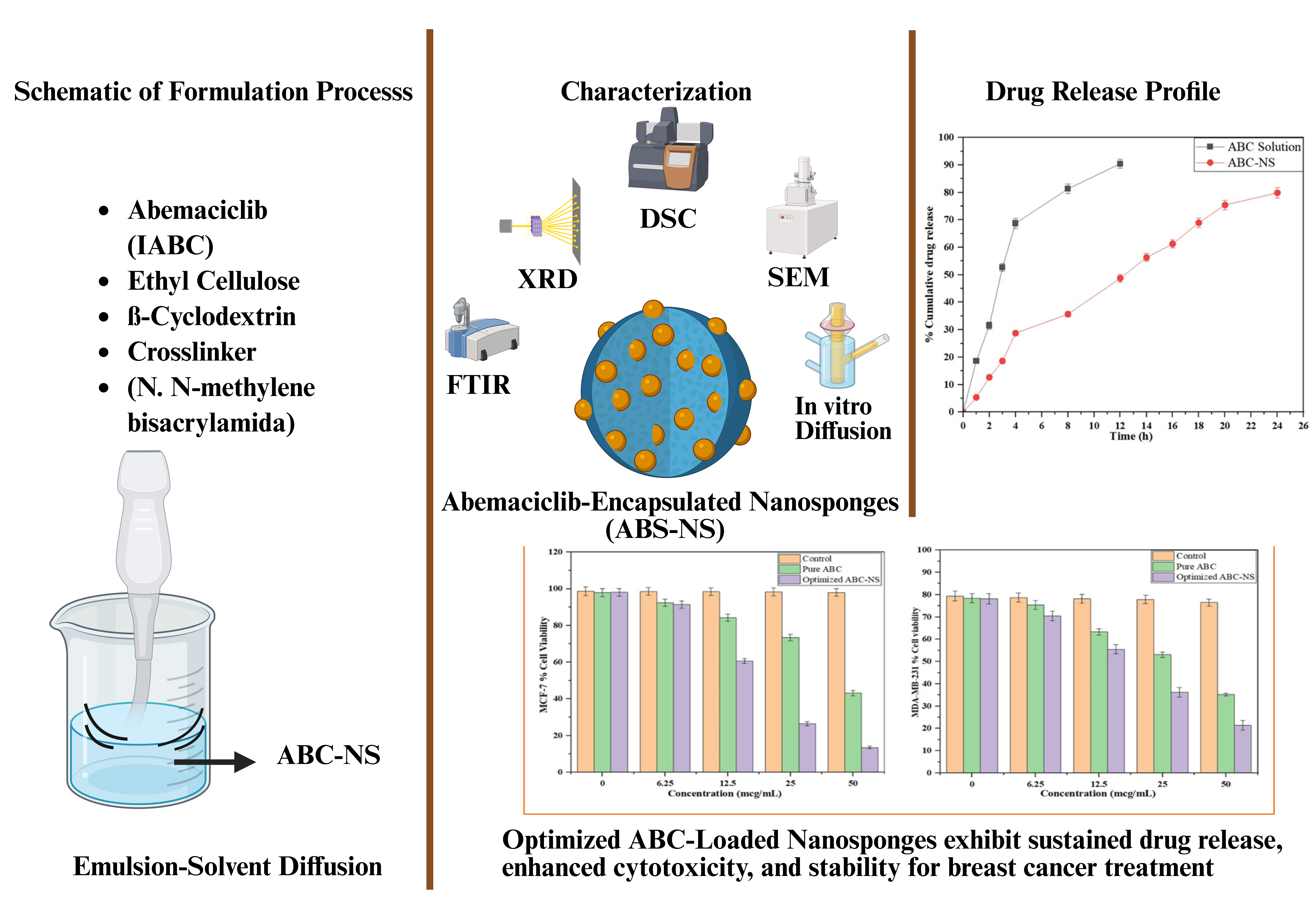
Background: Abemaciclib (ABC) is a new, orally administered pharmaceutical agent authorised for the purpose of combating breast cancer. The drug's low bioavailability necessitates dosing two to three times daily, which may reduce patient compliance. To lessen the severity of side effects and prolong the duration of action, sustained-release formulations are required. Developing an ABC sustained-release nanoparticle system was the primary goal of this study. Methodology: Both the sustained-release polymer (EC) and the surfactant (KP-188) were derived from ethyl cellulose, in an emulsion-solvent diffusion synthesis of nanosponges (NS). We examined the impact of varying surfactant concentrations and drug-to-polymer ratios on PS, PDI, ZP, %EE, %DL, particle size, drug loading, zeta potential, and polydispersity index. Results and Discussion: The optimized formulation (F11) achieved an entrapment efficiency of 86.52±0.25% and a cumulative drug release of 77.12% over 24 hours. The drug release followed a sustained pattern over 24 hours. It best fits the Higuchi kinetic model, which indicates that drug diffusion was the primary mechanism of release from the matrix system. The MTT experiment demonstrated that ABC might be a viable cytotoxic nanocarrier for breast cancer cells from humans, specifically MCF-7 and MDA-MB-231. On top of that, following contact with storage settings of 25, 5, and 45 °C for six months, ABC maintained its drug release property with no modification in the percentage release. Conclusion: This study shows that the created NS could effectively transport and release ABC, amplifying its impact in the battle against breast cancer.
Downloads
References
Obeagu EI, Obeagu GU. Breast cancer: A review of risk factors and diagnosis. Medicine, 103(3), e36905 (2024) http://dx.doi.org/10.1097/MD.0000000000036905
Houghton SC, Hankinson SE. Cancer progress and priorities: breast cancer. Cancer epidemiology, biomarkers and prevention, 30(5), 822-44 (2021) https://doi.org/10.1158/1055-9965.epi-20-1193
Giaquinto AN, Sung H, Newman LA, Freedman RA, Smith RA, Star J, Jemal A, Siegel RL. Breast cancer statistics. CA: a cancer journal for clinicians, 74(6), 477-95 (2024) https://doi.org/10.3322/caac.21863
Liu J, He M, Wang Z, Li Q, Xu B. Current research status of metronomic chemotherapy in combination treatment of breast cancer. Oncology research and treatment, 45(11), 681-92 (2022) https://doi.org/10.1159/000526481
Aramini B, Masciale V, Grisendi G, Bertolini F, Maur M, Guaitoli G, Chrystel I, Morandi U, Stella F, Dominici M, Haider KH. Dissecting tumor growth: the role of cancer stem cells in drug resistance and recurrence. Cancers, 14(4), 976 (2022) https://doi.org/10.3390/cancers14040976
Nelson BE, Adashek JJ, Lin SH, Subbiah V. On target methods to induce abscopal phenomenon for Off‐Target effects: From happenstance to happenings. Cancer medicine, 12(6), 6451-65 (2023) https://doi.org/10.1002/cam4.5454
Ding L, Cao J, Lin W, Chen H, Xiong X, Ao H, Yu M, Lin J, Cui Q. The roles of cyclin-dependent kinases in cell-cycle progression and therapeutic strategies in human breast cancer. International journal of molecular sciences, 21(6), 1960 (2020) http://dx.doi.org/10.3390/ijms21061960
Pellarin I, Dall’Acqua A, Favero A, Segatto I, Rossi V, Crestan N, Karimbayli J, Belletti B, Baldassarre G. Cyclin-dependent protein kinases and cell cycle regulation in biology and disease. Signal transduction and targeted therapy, 10(1), 11 (2025) https://doi.org/10.1038/s41392-024-02080-z
Tchakarska G, Sola B. The double dealing of cyclin D1. Cell cycle, 19(2),163-78 (2020) https://doi.org/10.1080/15384101.2019.1706903
Jeffreys SA, Becker TM, Khan S, Soon P, Neubauer H, de Souza P, Powter B. Prognostic and predictive value of CCND1/Cyclin D1 amplification in breast cancer with a focus on postmenopausal patients: a systematic review and meta-analysis. Frontiers in endocrinology, 13, 895729 (2022) https://doi.org/10.3389/fendo.2022.895729
Zhang M, Zhang L, Hei R, Li X, Cai H, Wu X, Zheng Q, Cai C. CDK inhibitors in cancer therapy, an overview of recent development. American journal of cancer research, 11(5), 1913 (2021) https://pmc.ncbi.nlm.nih.gov/articles/PMC8167670/
Mounika P, Gurupadayya B, Kumar HY, Namitha B. An overview of CDK enzyme inhibitors in cancer therapy. Current cancer drug targets, 23(8), 603-19 (2023) https://doi.org/10.2174/1568009623666230320144713
Asnaashari S, Amjad E, Sokouti B. Synergistic effects of flavonoids and paclitaxel in cancer treatment: a systematic review. Cancer cell international, 23(1), 211 (2023) https://doi.org/10.1186/s12935-023-03052-z
Gerosa R, De Sanctis R, Jacobs F, Benvenuti C, Gaudio M, Saltalamacchia G, Torrisi R, Masci G, Miggiano C, Agustoni F, Pedrazzoli P. Cyclin-dependent kinase 2 (CDK2) inhibitors and others novel CDK inhibitors (CDKi) in breast cancer: clinical trials, current impact, and future directions. Critical reviews in oncology/hematology, 104324 (2024) https://doi.org/10.1016/j.apsb.2020.05.001
Yuan K, Wang X, Dong H, Min W, Hao H, Yang P. Selective inhibition of CDK4/6: A safe and effective strategy for developing anticancer drugs. Acta pharmaceutica sinica B, 11(1), 30-54 (2021) http://dx.doi.org/10.2174/0115734137288288240108073034.
Goel S, Bergholz JS, Zhao JJ. Targeting CDK4 and CDK6 in cancer. Nature reviews cancer, 22(6), 356-72 (2022) https://doi.org/10.1038/s41568-022-00456-3
Ettl T, Schulz D, Bauer RJ. The renaissance of cyclin dependent kinase inhibitors. Cancers, 14(2), 293 (2022) https://doi.org/10.3390/cancers14020293
Fassl A, Geng Y, Sicinski P. CDK4 and CDK6 kinases: From basic science to cancer therapy. Science, 375(6577), eabc1495 (2022) https://doi.org/10.1126/science.abc1495
Piezzo M, Cocco S, Caputo R, Cianniello D, Gioia GD, Lauro VD, Fusco G, Martinelli C, Nuzzo F, Pensabene M, Laurentiis MD. Targeting cell cycle in breast cancer: CDK4/6 inhibitors. International journal of molecular sciences, 21(18), 6479 (2020) https://doi.org/10.3390/ijms21186479
Julve M, Clark JJ, Lythgoe MP. Advances in cyclin-dependent kinase inhibitors for the treatment of melanoma. Expert opinion on pharmacotherapy, 22(3), 351-61 (2020) https://doi.org/10.1080/14656566.2020.1828348
Saleh L, Wilson C, Holen I. CDK4/6 inhibitors in breast cancer–from in vitro models to clinical trials. Acta oncologica, 59(2), 219-32 (2022) https://doi.org/10.1080/0284186X.2019.1684559
Chaurasia M, Singh R, Sur S, Flora SJ. A review of FDA approved drugs and their formulations for the treatment of breast cancer. Frontiers in Pharmacology, 14, 1184472 (2023) https://doi.org/10.3389/fphar.2023.1184472
Arora S, Narayan P, Osgood CL, Wedam S, Prowell TM, Gao JJ, Shah M, Krol D, Wahby S, Royce M, Ghosh S. US FDA drug approvals for breast cancer: a decade in review. Clinical cancer research, 28(6), 1072-86 (2022) https://doi.org/10.1158/1078-0432.ccr-21-2600
Leo CP, Leo C, Szucs TD. Breast cancer drug approvals by the US FDA from 1949 to 2018. Nature reviews drug discovery, 19(11), (2020) https://doi.org/10.1038/d41573-019-00201-w
Łukasik P, Baranowska-Bosiacka I, Kulczycka K, Gutowska I. Inhibitors of cyclin-dependent kinases: types and their mechanism of action. International journal of molecular sciences, 22(6), 2806 (2021) https://doi.org/10.3390/ijms22062806
Mane PT, Wakure BS, Wakte PS. Cyclodextrin based nanosponges: a multidimensional drug delivery system and its biomedical applications. Current drug delivery, 18(10), 1467-93 (2021) https://doi.org/10.2174/1567201818666210423091250
Poulson BG, Alsulami QA, Sharfalddin A, El Agammy EF, Mouffouk F, Emwas AH, Jaremko L, Jaremko M. Cyclodextrins: Structural, chemical, and physical properties, and applications. Polysaccharides, 3(1), 1-31 (2021) https://doi.org/10.3390/polysaccharides3010001
Pyrak B, Rogacka-Pyrak K, Gubica T, Szeleszczuk Ł. Exploring cyclodextrin-based nanosponges as drug delivery systems: Understanding the physicochemical factors influencing drug loading and release kinetics. International journal of molecular sciences, 25(6), 3527 (2024) https://doi.org/10.3390/ijms25063527
Kapoor DU, Garg R, Saini PK, Gaur M, Prajapati BG. Nanomedicine breakthrough: Cyclodextrin-based nano sponges revolutionizing cancer treatment. Nano-structures and nano-objects, 40, 101358 (2024) https://doi.org/10.1016/j.nanoso.2024.101358
Wadhwa P, Vij M, Dand N. Wave-assisted techniques, a greener and quicker alternative to synthesis of cyclodextrin-based nanosponges: A review. Recent patents on nanotechnology, 18(2), 207-19 (2024) https://doi.org/10.2174/1872210516666220928114103
Al-Shdefat R, Hailat M, Alshogran OY, Abu Dayyih W, Gardouh A, Al Meanazel O. Ribociclib hybrid lipid–polymer nanoparticle preparation and characterization for cancer treatment. Polymers. 15(13), 2844 (2023) https://doi.org/10.3390/polym15132844
Shah, H. S., Zaib, S., Khan, I., Sliem, M. A., Alharbi, O., Al-Ghorbani, M., ... & Awan, S.. Preparation and investigation of a novel combination of Solanum nigrum-loaded, arabinoxylan-cross-linked β-cyclodextrin nanosponges for the treatment of cancer: in vitro, in vivo, and in silico evaluation. Frontiers in Pharmacology, 14, 1325498 (2023) https://doi.org/10.3389/fphar.2023.1325498.
Aboushanab AR, El-Moslemany RM, El-Kamel AH, Mehanna RA, Bakr BA, Ashour AA. Targeted fisetin-encapsulated β-cyclodextrin nanosponges for breast cancer. Pharmaceutics. 15(5), 1480 (2023) https://doi.org/10.3390/pharmaceutics15051480
Mane PT, Wakure BS, Wakte PS. Enhancement in the therapeutic potential of lapatinib ditosylate against breast cancer by the use of β-cyclodextrin based ternary nanosponge system. International journal of pharmaceutics, 642, 123210 (2023) https://doi.org/10.1016/j.ijpharm.2023.123210
Rajalakshmi P, Peter DN, S JJ, Ananthi N. Structure-activity relationship of supramolecular compounds in drug delivery. Mini-reviews in organic chemistry, 18(7), 961-89 (2021) http://dx.doi.org/10.2174/1570193X17999201123212536
Tiwari K, Bhattacharya S. The ascension of nanosponges as a drug delivery carrier: preparation, characterization, and applications. Journal of Materials Science: Materials in medicine, 33(3), 28 (2022) https://doi.org/10.1007/s10856-022-06652-9
Avula PR, Chettupalli AK, Chauhan V, Jadi RK. Design, formulation, in-vitro and in-vivo pharmacokinetic evaluation of Nicardipine-nanostructured lipid carrier for transdermal drug delivery system. Materials today proceedings, (2023) https://doi.org/10.1016/j.matpr.2023.06.282
Chettupalli AK, Ajmera S, Amarachinta PR, Manda RM, Jadi RK. Quality by Design approach for preparation, characterization, and statistical optimization of naproxen sodium-loaded ethosomes via transdermal route. Current bioactive compounds, 19(10), 79-98 (2023) https://doi.org/10.2174/1573407219666230606142116
Amarachinta PR, Sharma G, Samed N, Chettupalli AK, Alle M, Kim JC. Central composite design for the development of carvedilol-loaded transdermal ethosomal hydrogel for extended and enhanced anti-hypertensive effect. Journal of nanobiotechnology, 19, 1–5 (2021) https://doi.org/10.1186/s12951-021-00833-4
Sharaff CS, Renukuntla P, Peddapalli H, Kuchukuntla M, Bakshi V, Jadi RK. Formulation, development, and characterization of loratadine emulgel. Journal of applied pharmaceutical research, 12(2), 42-50 (2024) https://doi.org/10.18231/j.joapr.2024.12.2.42.50
Chettupalli AK, Rao PA, Kuchukuntla M, Bakshi V. Development and Optimization of Aripiprazole ODT by using box-Behnken Design. Research journal of pharmacy and technology, 13(12), 6195–201 (2020) https://doi.org/10.5958/0974-360X.2020.01080.X
Chettupalli AK, Unnisa A, Peddapalli H, Jadi RK, Anusha K, Amarachinta PR. Development and evaluation of empagliflozin-loaded solid lipid nanoparticles: Pharmacokinetics and pharmacodynamics for oral delivery. Intelligent pharmacy, (2025) https://doi.org/10.1016/j.ipha.2024.12.004
Chettupalli AK, Ajmera S, Kuchukuntla M, Palanivel V, Katta S. Design Formulation of Nanospanlastic Novel Carriers as a Promising Approach to Enhanced Bioavailability in Intranasal Drug Delivery for Sinusitis: Statistical Optimization and In vitro and In vivo Characterization. Current nanomedicine (Formerly: Recent patents on nanomedicine), 14(3), 266-88 (2024) https://doi.org/10.2174/0124681873262019231105201433
Prasad RR, Kumar JR, Vasudha BA, Kumar CA. Formulation development and evaluation of allopurinol solid dispersions by solvent evaporation technique. International joural of applied pharmaceutics, 10(4), 168–71 (2018) http://dx.doi.org/10.22159/ijap.2018v10i4.25311
Unnisa A, Chettupalli AK, Alazragi RS, Alelwani W, Bannunah AM, Barnawi J, Amarachinta PR, Jandrajupalli SB, Elamine BA, Mohamed OA, Hussain T. Nanostructured lipid carriers to enhance the bioavailability and solubility of ranolazine: Statistical optimization and pharmacological evaluations. Pharmaceuticals, 16(8), 1151 (2023) https://doi.org/10.3390/ph16081151
Sameina LH, Idamakantia S, Chettupalli AK, Velamala RR, Ezzat MO. Design of mesalamine loaded micro-particles: Preparation, in-vitro and in-vivo characterization. Materials today: proceedings, (2023) https://doi.org/10.1016/j.matpr.2023.07.063
Chettupalli AK, Avula PR, Chauhan V. Improved Transdermal Delivery of Anti-hypertensive Drug Loaded Nanostructured Lipid Carriers: Statistical Design, Optimization, Depiction and Pharmacokinetic Assessment. Current drug therapy, 19(7), 828–45 (2024) https://doi.org/10.2174/01157488552678312311131124451
Published
How to Cite
Issue
Section
Copyright (c) 2025 Nirosha Bolledla, Vasudha Bakshi

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
















