Pharmacognostical, phytochemical, and in vitro bioassay studies of Osbeckia stellata Buch-ham. leaves
DOI:
https://doi.org/10.69857/joapr.v13i4.1199Keywords:
Osbeckia stellata, Pharmacognosy, Phytochemistry, In vitro studies, StandardizationAbstract
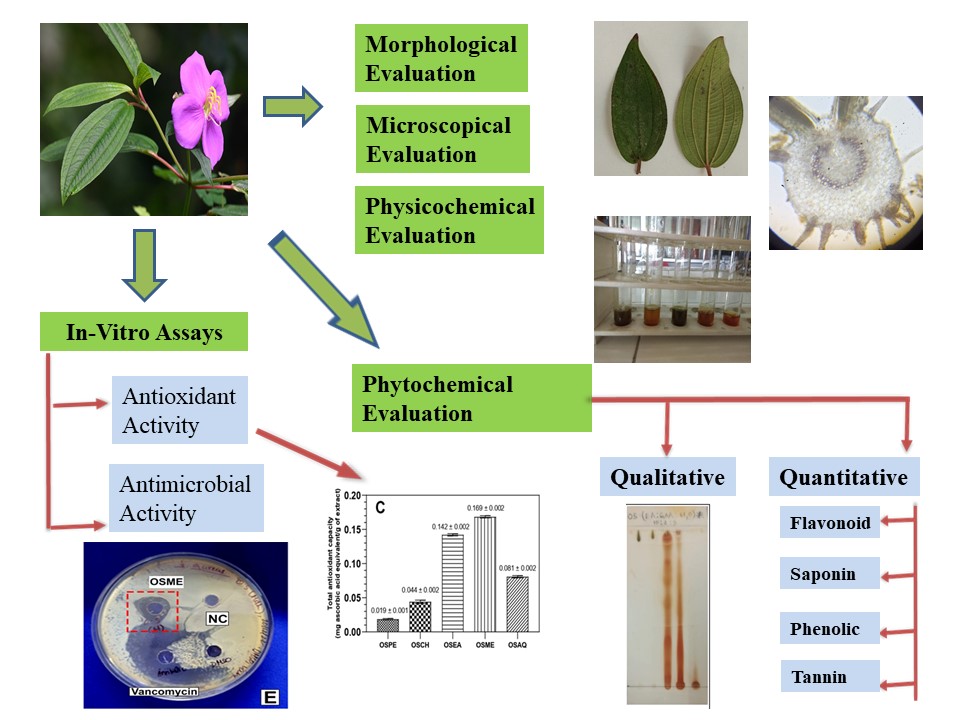
Background: Osbeckia stellata (Os) is a medicinally significant herb that is consumed for the treatment of various diseases, including skin diseases, diabetes, diarrhea, cancer, asthma, arthritis, dysentery, leukoderma, hypertension, jaundice, malaria, rheumatism, spondylitis, and tuberculosis, as well as inflammation and wound healing. Methodology: This study standardizes the plant of Os by accepted practices. Os leaves have been examined physicochemically, phytochemically, microscopically, and morphologically. Extracts were reviewed for both qualitative and quantitative phytochemical examination, and in vitro bioassays were also evaluated. Results: Diagnostic traits, such as xylem arteries, trichomes with cover, and anomocytic stomata, were identified in the histological study. Nutritional profiling revealed fiber content (48.1 ± 0.99 mg/100 g). Heavy metal analysis revealed that Pb, Hg, Sn, Sb, Cd, Cu, and As were within the permissible limits. Pesticide residues were verified with ICP-MS analysis. The in vitro antioxidant studies of different extracts show IC₅₀ values 1003.35±0.23, 152.11±0.1, 192.12±0.14, 111.79±0.06, and 982.49±0.31 (μg/ml) as compared to standard 130.54±0.03 and 330.86±0.09 (μg/ml). Antimicrobial assay studies show the Zone of Inhibition by different extracts is 26.00 ± 1.20, 17.00 ± 0.60, 18.66 ± 0.58, 22.33 ± 1.52, 6.33 ± 0.58 (mm) as compared to the standard 38.00 ± 1.00, 35.00 ± 1.35, 22.00 ± 1.00, 41.00 ± 1.00, 30.66 ± 1.54(mm). Discussion: The methanol extract of Os has total phenols and total tannins of 120.04±5.97 and 123.0±1.52 (mg/g TAE), respectively, which is high in quantity and is reported to possess high antimicrobial and antioxidant properties. Conclusion: This study concludes that the quality control parameters for Os are essential for promoting its use in pharmaceutical applications.
Downloads
References
Pulpra P, Thomas SM. The genus Osbeckia (Melastomataceae) in India. Journal of the Indian Association for Angiosperm Taxonomy, 29(4), 236–305 (2019) https://doi.org/10.22244/rheedea.2019.29.4.01
Pulpra P. The genus Osbeckia (Melastomataceae) in India. Rheedea, 29, 1-3 (2019) https://doi.org/10.22244/rheedea.2019.29.4.01
Prasadani M, Bogahawaththa S, Illeperuma RP, Kodithuwakku SP. Leaf Extract of Osbeckia octandra L. (Heen Bovitiya) Suppresses Human Oral Squamous Cell Carcinoma Cells Migration and Induces Cellular DNA Damage. Journal of Oral and Maxillofacial Surgery, Medicine, and Pathology, 33(2), 215-220 (2021) https://doi.org/10.1016/j.ajoms.2020.09.003
Grayer RJ, Thabrew MI, Hughes RD, Bretherton S, Lever A, Veitch, NC. Phenolic and Terpenoid Constituents from the Sri Lankan Medicinal Plant Osbeckia aspera. Pharma. Biol., 46(3), 154 – 161 (2008) https://doi.org/10.1080/13880200701538682
Gurumayum S, Bharadwaj S, Sheikh Y, Barge SR, Saikia K, Swargiary D et al. Taxifolin-3-O-glucoside from Osbeckia nepalensis Hook. mediates antihyperglycemic activity in CC1 hepatocytes and in diabetic Wistar rats via regulating AMPK/G6Pase/PEPCK signaling axis. J Ethnopharmacol, 303, 115936 (2023) https://doi.org/10.1016/j.jep.2022.115936
Priya A, Das SP, Goswami S, Adak MK, Deb D, Dey N. An Exploratory Study on Allelic Diversity for Five Genetic Loci Associated with Floral Organ Development in Rice. American Journal of Plant Sciences, 6, 12 (2015) https://doi.org/10.4236/ajps.2015.612198
Baishya T, Das P, Ashraf GJ, Dua TK, Paul P, Nandi G et al. Antioxidant activity, cytotoxicity assay, and evaluation of bioactive compounds using GC-MS and HPTLC-based bioassay in the extracts of Osbeckia stellata var. crinita (Benth. ex Naudin) grown in Manipur, India. Kuwait Journal of Science, 51(3), 100229 (2024) https://doi.org/10.1016/j.kjs.2024.100229
Ling C, Zhang M, Ma W, Liu M. The complete sequence of chloroplast genome of Baeckea frutescens Linaeus 1753 (Myrtoideae), a traditional folk medicinal plant. Mitochondrial DNA B Resour, 9(10), 1384-1388 (2024) https://doi.org/10.1080/23802359.2024.2412239
Liang R, Ruan F, Zhi X, & Zhou Q. Characterization of the chloroplast genome of Osbeckia stellata (Melastomataceae). Mitochondrial DNA Part B, 4(1), 1136–1137 (2019) https://doi.org/10.1080/23802359.2019.1574679
Balami NP. Ethnomedicinal uses of plants among the Newar community of Pharping village of Kathmandu distinct, Nepal. Tribhuvan Univ J., 24, 13–19 (2004) https://doi.org/10.3126/tuj.v24i1.251
Saravanan R, Das M. Medicinal plants industry in India: Challenges, opportunities and sustainability. Medicinal Plants - International Journal of Phytomedicines and Related Industries, 16, 1-14 (2024) https://doi.org/10.5958/0975-6892.2024.00001.7
Bordoloi C, Kumar S, Barbhuiya AM, Kushari S, Kalita JM, Sahu BP et al. Herbal medicine used for wound healing by the tribes of the North Eastern states of India: a comprehensive review. Journal of Herbal Medicine, 41, 100697 (2023) https://doi.org/10.1016/j.hermed.2023.100697
Shen X, Zhou M, Zhu X, Zhang J, Xu J, Jiang W. Chemical composition and antioxidant activity of petroleum ether fraction of Rosmarinus officinalis. Heliyon, 9(11), e21316 (2023) https://doi.org/10.1016/j.heliyon.2023.e21316
Yang Y, Moh SH, Yu T, Park JG, Yoon DH, Kim TW et al. Methanol extract of Osbeckia stellata suppresses lipopolysaccharide- and HCl/ethanol-induced inflammatory responses by inhibiting Src/Syk and IRAK1. Journal of Ethnopharmacology, 143(3), 876-883 (2012) https://doi.org/10.1016/j.jep.2012.08.015
Gangaram S, Naidoo Y, Dewir YH, Singh M, Lin J, Hosakatte NM. Phytochemical Composition and Antibacterial Activity of Barleria albostellata C.B. Clarke Leaf and Stem Extracts. Plants, 12(13), 2396 (2023) https://doi.org/10.3390/plants12132396
Laloo D, Kumar M, Prasad SK, Hemalatha S. Quality control standardization of the roots of Potentilla fulgens Wall.: A potent medicinal plants of the Western Himalayas and Northeastern India. Pharmacognosy Journal, 5(3), 97-103 (2013) https://doi.org/10.1016/j.phcgj.2013.04.002
Fernández V, Bahamonde HA, Peguero-Pina JJ, Gil-Pelegrín E, Sancho-Knapik D, Gil L et al. Physico-chemical properties of plant cuticles and their functional and ecological significance. Journal of Experimental Botany, 68(19), 5293–5306 (2017) https://doi.org/10.1093/jxb/erx302
Jeevitha M, Ravi PV, Subramaniyam V, Moorthi P, Sripathi SK. Exploring the phyto- and physicochemical evaluation, fluorescence characteristics, and antioxidant activities of Acacia ferruginea Dc: an endangered medicinal plant. Futur J Pharm Sci, 7, 228 (2021) https://doi.org/10.1186/s43094-021-00375-4
Liang G, Gong W, Li B, Zuo J, Pan L, Liu X. Analysis of Heavy Metals in Foodstuffs and an Assessment of the Health Risks to the General Public via Consumption in Beijing, China. Int J Environ Res Public Health, 16(6), 909 (2019) https://doi.org/10.3390/ijerph16060909
Tokaliioglu S. Determination of trace elements in commonly consumed medicinal herbs by ICP-MS and multivariate analysis. Food Chem, 134, 2504-2508 (2012) https://doi.org/10.1016/j.foodchem.2012.04.093
Narenderan ST, Meyyanathan SN, Babu B. Review of pesticide residue analysis in fruits and vegetables. Pre-treatment, extraction and detection techniques. Food Research International, 133, 109141 (2020) https://doi.org/10.1016/j.foodres.2020.109141
Laloo D, Tahbildar R, Smith K, Ahmed AU, Nath J, Shil D et al. Impact of quality control standardization parameters and antioxidant potential of the aerial parts of Potentilla fulgens Wall.: a comprehensive monographic study. J Biologically Act Prod Nat, 10(4), 338-356 (2020) https://doi.org/10.1080/22311866.2020.1806731
Kalemba MRK, Makhuvele R, Njobeh PB. Phytochemical screening, antioxidant activity of selected methanolic plant extracts and their detoxification capabilities against AFB1 toxicity. Heliyon, 10(2), e24435 (2024) https://doi.org/10.1016/j.heliyon.2024.e24435
Le BM, Thibault J, Pottier Q, Boisard S, Guilet D. An accurate, cost-effective and simple colorimetric method for the quantification of total triterpenoid and steroidal saponins from plant materials. Food Chem, (30)383, 132597 (2022) https://doi.org/10.1016/j.foodchem.2022.132597
Hayat J, Akodad M, Moumen A, Baghour M, Skalli A, Ezrari S et al. Phytochemical screening, polyphenols, flavonoids and tannin content, antioxidant activities and FTIR characterization of Marrubium vulgare L. from 2 different localities of Northeast of Morocco. Heliyon, 6(11), e05609 (2020) https://doi.org/10.1016/j.heliyon.2020.e05609
Roy A, Khan A, Ahmad I, Alghamdi S, Rajab BS, Babalghith AO et al. Flavonoids a Bioactive Compound from Medicinal Plants and Its Therapeutic Applications. Biomed Res Int, 6, 5445291 (2022) https://doi.org/10.1155/2022/5445291
Timilsena YP, Phosanam A, Stockmann R. Perspectives on Saponins: Food Functionality and Applications. Int J Mol Sci, 24(17), 13538 (2023) https://doi.org/10.3390/ijms241713538
Baliyan S, Mukherjee R, Priyadarshini A, Vibhuti A, Gupta A, Pandey RP et al. Determination of Antioxidants by DPPH Radical Scavenging Activity and Quantitative Phytochemical Analysis of Ficus religiosa. Molecules, 27(4), 1326 (2022) https://doi.org/10.3390/molecules27041326
Jafri L, Saleem S, Haq I, Ullah N, Mirza B. In vitro assessment of antioxidant potential and determination of polyphenolic compounds of Hedera nepalensis K. Koch. Arabian Journal of Chemistry, 10(2), S3699-S3706 (2017) https://doi.org/10.1016/j.arabjc.2014.05.002
Gangaram S, Naidoo Y, Dewir YH, Singh M, Lin J, Murthy HN. Phytochemical Composition and Antibacterial Activity of Barleria albostellata C.B. Clarke Leaf and Stem Extracts. Plants, 12(13), 2396 (2023) https://doi.org/10.3390/plants12132396
Gulcin İ, Alwasel SH. Fe3+ Reducing Power as the Most Common Assay for Understanding the Biological Functions of Antioxidants. Processes, 13(5), 1296 (2025) https://doi.org/10.3390/pr13051296
Manandhar S, Luitel S, Dahal RK. In vitro antimicrobial activity of some medicinal plants against Human Pathogenic Bacteria. Hindawi Journal of Tropical Medicine, 1895340, 1-5 (2019) https://doi.org/10.1155/2019/1895340
Kumari N, Mittal A, Rana A, Sharma AK. Identification of different extracts and phytoconstituents of Callistemon viminalis Cheel for their anti-anxiety effects based on pharmacognostic, toxicological, and pharmacological strategies. Toxicol Rep, 12(13), 101726 (2024) https://doi.org/10.1016/j.toxrep.2024.101726
Vignesh A, Amal TC, Sarvalingam A, Vasanth K. A review on the influence of nutraceuticals and functional foods on health. Food Chemistry Advances, 5, 100749 (2024) https://doi.org/10.1016/j.focha.2024.100749
Pandey M, AlQassab O, Kanthajan T, Parikh A, Francis AJ, Sreenivasan C et al. Effectiveness of High-Fiber, Plant-Based Diets in Reducing Cardiovascular Risk Factors Among Middle-Aged and Older Adults: A Systematic Review. Cureus, 16(8), e67660 (2024) https://doi.org/10.7759/cureus.67660
Prasad RS, Yenorkar NY, Dhaswadikar SR, Sinha SK, Rai N, Sharma P et al. A systematic antidiarrheal evaluation of a vegetable root Begonia roxburghii and its marker flavonoids against nonpathogenic and pathogenic diarrhea. Food Bioscience, 53, 102672 (2023) https://doi.org/10.1016/j.fbio.2023.102672
Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN. Toxicity, mechanism and health effects of some heavy metals. Interdisciplinary Toxicology, 7(2), 60-72 (2014) https://doi.org/10.2478/intox-2014-0009
He S, Niu Y, Xing L, Liang Z, Song X, Ding M et al. Research progress of the detection and analysis methods of heavy metals in plants. Front Plant Sci., 31(15), 1310328 https://doi.org/10.3389/fpls.2024.1310328
Dubey P, Thakur V, Chattopadhyay M. Role of minerals and trace elements in diabetes and insulin resistance. Nutrients, 12, 1864 (2020) https://doi.org/10.3390/nu12061864
Zagoskina NV, Zubova MY, Nechaeva TL, Kazantseva VV, Goncharuk EA, Katanskaya VM et al. Polyphenols in Plants: Structure, Biosynthesis, Abiotic Stress Regulation, and Practical Applications (Review). Int J Mol Sci, 24(18), 13874 (2023) https://doi.org/10.3390/ijms241813874
Laloo D, Sahu AN, Hemalatha S, Dubey SD. Pharmacognostical and phytochemical evaluation of Cinnamomum wightii Meissen. Flowers. Indian Journal of Natural Products and Resources, 3(1), 33-39 (2012) https://doi.org/10.5530/pj.2019.1.15
Oleszek W, Kapusta I, Stochmal A. TLC of Triterpenes (Including Saponins). Hajnos/Thin Layer Chromatography in Phytochemistry. TSuresh Publisher, Poland (2014) https://doi.org/10.1201/9781420046786.ch20
Oalđe PM, Kolarević S, Đorđević AJ, Vuković-Gačić B. Exploring the Antibacterial Potential of Lamiaceae Plant Extracts: Inhibition of Bacterial Growth, Adhesion, Invasion, and Biofilm Formation and Degradation in Pseudomonas aeruginosa PAO1. Plants (Basel), 13(12), 1616 (2024) https://doi.org/10.3390/plants13121616
Hamdi AM, Abbas AI, Munis DM. Antimicrobial Resistance of Tannin Extract against E. coli Isolates from Sheep. Arch Razi Inst, 77(2), 697-701 (2022) https://doi.org/10.22092/ARI.2022.356982.1955
Published
How to Cite
Issue
Section
Copyright (c) 2025 Chayanika Bordoloi, Nilutpal Sharma Bora, Damiki Laloo

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
















