Design and optimization of folate-targeted lipid-polymer hybrid nanoparticles co-encapsulating dexamethasone and curcumin for synergistic anti-inflammatory efficacy in rheumatoid arthritis
DOI:
https://doi.org/10.69857/joapr.v13i5.1176Keywords:
Rheumatoid Arthritis, Folate-targeted Lipid-polymer hybrid nanoparticles, Dexamethasone, Curcumin, Nanocarrier optimization, Box Behnken Design, RAW 264.7 macrophage cellsAbstract
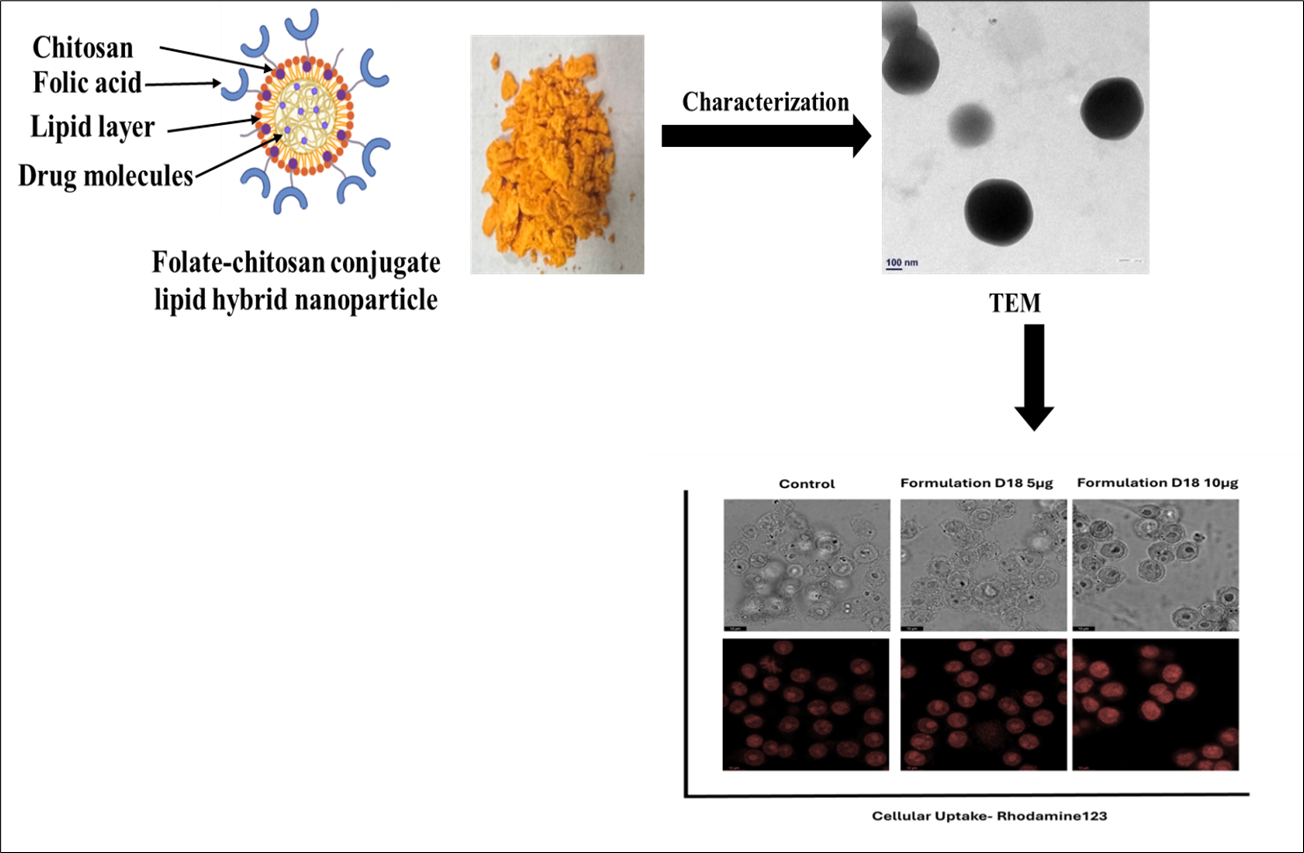
Background: Rheumatoid Arthritis (RA) is a chronic immune-mediated disorder characterized by synovial inflammation and joint destruction. Current therapies are limited by systemic toxicity and poor bioavailability. This research developed Dexamethasone (Dex) and Curcumin (Cur) loaded Folate Lipid Polymer Hybrid Nanoparticles (DCFLPs) to achieve synergistic anti-inflammatory action for RA. Methodology: DCFLPs were synthesized by the ionic gelation technique. Furthermore, Box-Behnken Design (BBD) formulations were optimized and evaluated for size distribution, PDI, ζ potential, structural features, % encapsulation efficiency (EE), in vitro release profile, and cell line studies using RAW 264.7 cells. Results and Discussion: Optimized DCFLPs revealed an average particle size of 287.8 ± 1.32 nm and PDI 0.25 with positive ζ potential 5.4 mV, and have shown high entrapment efficiencies for Dex (89.12 ± 0.087%) and Cur (98.27± 0.110%). Cytotoxicity assays showed superior anti-inflammatory activity, and enhanced cellular uptake was observed in cell line studies. Conclusion: DCFLPs offer an auspicious approach for targeted RA therapy by combining controlled drug release, reduced systemic toxicity, and enhanced site-specific delivery. These findings suggest that the synthesized formulation has the potential to serve as a viable approach for in vivo translation, future preclinical evaluation, and effective progression towards clinical application in RA management.
Downloads
References
Xu Y, Zhao M, Cao J, Fang T, Zhang J, Zhen Y, Wu F, Yu X, Liu Y, Li J, Wang D. Applications and recent advances in transdermal drug delivery systems for the treatment of rheumatoid arthritis. Acta Pharm. Sin. B, 13, 4417-41 (2023) https://doi.org/10.1016/j.apsb.2023.05.025.
Cai Y, Zhang J, Liang J, Xiao M, Zhang G, Jing Z, Lv L, Nan K, Dang X. The Burden of Rheumatoid Arthritis: Findings from the 2019 Global Burden of Diseases Study and Forecasts for 2030 by Bayesian Age-Period-Cohort Analysis. J. Clin. Med., 12, 1291 (2023) https://doi.org/10.3390/jcm12041291.
Singh S, Tiwary N, Sharma N, Behl T, Antil A, Anwer MK, Ramniwas S, Sachdeva M, Elossaily GM, Gulati M, Ohja S. Integrating Nanotechnological Advancements of Disease-Modifying Anti-Rheumatic Drugs into Rheumatoid Arthritis Management. Pharmaceuticals, 17, 248 (2024) https://doi.org/10.3390/ph17020248.
Khan S, Mohan K, Muzammil S, Alam MA, Khayyam KU. Current Prospects in Rheumatoid Arthritis: Pathophysiology, Genetics, and Treatments. Recent Adv. Antiinfect. Drug Discov., 19, 36-55 (2024) https://doi.org/10.2174/2772434418666230406083149.
Li J, Li W, Zhuang L. Natural biomimetic nano-system for drug delivery in the treatment of rheumatoid arthritis: a literature review of the last 5 years. Front. Med., 11, 1385123 (2024) https://doi.org/10.3389/fmed.2024.1385123.
Bahmani A, Taghvaei A, Firozian F, Chehardoli G. Folic acid as an exploiter of natural endocytosis pathways in drug delivery. Chem. Methodol., 8, 96-122 (2024) https://doi.org/10.48309/CHEMM.2024.430060.1746.
Chandrupatla, D. M. S. H., Molthoff, C. F. M., Lammertsma, A. A., van der Laken, C. J., & Jansen, G. (2019). The folate receptor β as a macrophage-mediated imaging and therapeutic target in rheumatoid arthritis. Drug Deliv. Transl. Res., 9, 366–378 (2019) https://doi.org/10.1007/s13346-018-0589-2.
Li C, Luo X, Qian C, Huang J, Yi X, Su H, Han Y. Folate receptor-mediated targeted therapy for rheumatoid arthritis by methotrexate-phospholipid complex nano-emulsions. J. Drug Target., 31, 402-410 (2023) https://doi.org/10.1080/1061186X.2023.2175832.
Nasra S, Bhatia D, Kumar A. Targeted Macrophage Re-Programming: Synergistic Therapy With Methotrexate and RELA siRNA Folate-Liposome in RAW264.7 Cells and Arthritic Rats. Adv. Healthc. Mater., 13, 2400679 (2024) https://doi.org/10.1002/adhm.202400679.
Guadarrama-Escobar OR, Serrano-Castañeda P, Anguiano-Almazán E, Vázquez-Durán A, Peña-Juárez MC, Vera-Graziano R, Morales-Florido MI, Rodriguez-Perez B, Rodriguez-Cruz IM, Miranda-Calderón JE, Escobar-Chávez JJ. Chitosan Nanoparticles as Oral Drug Carriers. Int. J. Mol. Sci., 24, 4289 (2023) https://doi.org/10.3390/ijms24054289.
Jha R, Mayanovic RA. A Review of the Preparation, Characterization, and Applications of Chitosan Nanoparticles in Nanomedicine. Nanomaterials, 13, 1302 (2023) https://doi.org/10.3390/nano13081302.
Al-Nemrawi N, Wahsheh Y, Alzoubi KH. Transdermal Delivery of Methotrexate Loaded in Chitosan Nanoparticles to Treat Rheumatoid Arthritis. Curr. Drug Deliv., 21, 451-460 (2024) https://doi.org/10.2174/1567201820666230428124346.
Naseer RD, Muhammad F, Aslam B, Faisal MN. Anti-arthritic effects of geranium essential oil loaded chitosan nanoparticles in Freund's complete adjuvant induced arthritic rats through down-regulation of inflammatory cytokines. Inflammopharmacology, 31, 1893-1912 (2023) https://doi.org/10.1007/s10787-023-01233.
Dong J, Zhou X, Li Q, Zheng R, Chen J, Liu Y, Tong X, Wan Z, Gong T. The Advances in Phospholipids-Based Phase Separation Gels for the Sustained Release of Peptides, Proteins, and Chemotherapeutics. Pharmaceutics, 16, 875 (2024) https://doi.org/10.3390/pharmaceutics16070875.
Jain S, Kumar M, Kumar P, Verma J, Rosenholm JM, Bansal KK, Vaidya A. Lipid–polymer hybrid nanosystems: a rational fusion for advanced therapeutic delivery. J. Funct. Biomater., 14, 437 (2023) https://doi.org/10.3390/jfb14090437.
Zhang J, Yang J, Yu Z, Bai H, Wang Y, Wang R. DS-Modified Paeoniflorin pH-Responsive Lipid–Polymer Hybrid Nanoparticles for Targeted Macrophage Polarization in a Rat Model of Rheumatoid Arthritis. International Journal of Nanomedicine, 31, 8967-92 (2025) https://doi.org/10.2147/IJN.S516434.
Mrid RB, Bouchmaa N, Ainani H, El Fatimy R, Malka G, Mazini L. Anti-rheumatoid drugs advancements: New insights into the molecular treatment of rheumatoid arthritis. Biomedicine & Pharmacotherapy, 151, 113126 (2022) https://doi.org/10.1016/j.biopha.2022.113126.
Zhang Y, Zhou X, Wang Z, Wu M, Zhang W, Zhang Z, Sun X, Gong T. Dexamethasone Palmitate Encapsulated in Palmitic Acid Modified Human Serum Albumin Nanoparticles for the Treatment of Rheumatoid Arthritis. J. Pharm. Sci., 113, 2851-2860 (2024) https://doi.org/10.1016/j.xphs.2024.07.013.
Kim SJ, Choi Y, Min KT, Hong S. Dexamethasone-Loaded Radially Mesoporous Silica Nanoparticles for Sustained Anti-Inflammatory Effects in Rheumatoid Arthritis. Pharmaceutics, 14, 985 (2022) https://doi.org/10.3390/pharmaceutics14050985.
Fuloria S, Mehta J, Chandel A, Sekar M, Rani NNIM, Begum MY, Subramaniyan V, Chidambaram K, Thangavelu L, Nordin R, Wu YS, Sathasivam KV, Lum PT, Meenakshi DU, Kumarasamy V, Azad AK, Fuloria NK. A Comprehensive Review on the Therapeutic Potential of Curcuma longa Linn. in Relation to its Major Active Constituent Curcumin. Front Pharmacol., 13, 820806 (2022) https://doi.org/10.3389/fphar.2022.820806.
Asif HM, Zafar F, Ahmad K, Iqbal A, Shaheen G, Ansari KA, Rana S, Zahid R, Ghaffar S. Synthesis, characterization and evaluation of anti-arthritic and anti-inflammatory potential of curcumin loaded chitosan nanoparticles. Sci. Rep., 13, 10274 (2023) https://doi.org/10.1038/s41598-023-37152-7.
Bideshki MV, Jourabchi‐Ghadim N, Radkhah N, Behzadi M, Asemani S, Jamilian P, Zarezadeh M. The efficacy of curcumin in relieving osteoarthritis: A meta‐analysis of meta‐analyses. Phytotherapy Research., 38, 2875-91 (2024) https://doi.org/10.1002/ptr.8153.
Yan F, Li H, Zhong Z, Zhou M, Lin Y, Tang C, Li C. Co-delivery of prednisolone and curcumin in human serum albumin nanoparticles for effective treatment of rheumatoid arthritis. International Journal of Nanomedicine., 22, 9113-25 (2019) https://doi.org/10.2147/IJN.S219413.
Ponnusamy C, Krishnaswami V, Pandian S, Natesan S. In-vitro Drug Release and Ex-vivo Corneal Permeation of Dexamethasone and Curcumin Nanodispersions Using Facilitated Simultaneous RP-HPLC Technique. Analytical Chemistry Letters., 8, 311- 20 (2018) https://doi.org/10.1080/22297928.2018.1445555.
Patil N, Mahajan H. Development and validation of RP-HPLC method for simultaneous qualitative and quantitative estimation of curcumin and quercetin in bulk mixture. Indian J Pharm Educ. Res., 56 ,247-54 (2022) https://doi.org/10.5530/ijper.56.1.29
Aslam B, Hussain A, Faisal MN, Sindhu ZU, Khan RU, Alhidary IA, Naz S, Tufarelli V. Curcumin Co-Encapsulation Potentiates Anti-Arthritic Efficacy of Meloxicam Biodegradable Nanoparticles in Adjuvant-Induced Arthritis Animal Model. Biomedicines., 11, 2662 (2023) https://doi.org/10.3390/biomedicines11102662.
Shahzad A, Arshad S, Zubair F, Shahzad S, Batool F, Fu Q. Development and Validation of Facile RP-HPLC Method for Simultaneous Determination of Timolol Maleate, Moxifloxacin Hydrochloride, Diclofenac Sodium and Dexamethasone in Plasma, Aqueous Humor and Pharmaceutical Products. J Chromatogr. Sci., 61, 678-687 (2023) https://doi.org/10.1093/chromsci/bmac057.
Ullah S, Azad AK, Nawaz A, Shah KU, Iqbal M, Albadrani GM, Al-Joufi FA, Sayed AA, Abdel-Daim MM. 5-fluorouracil-loaded folic-acid-fabricated chitosan nanoparticles for site-targeted drug delivery cargo. Polymers., 14, 2010 (2022) https://doi.org/10.3390/polym14102010.
Kumbhar ST, Patil RY, Bhatia MS, Choudhari PB, Gaikwad VL. Synthesis and characterization of chitosan nanoparticles decorated with folate and loaded with dasatinib for targeting folate receptors in cancer cells. OpenNano., 7, 100043 (2022) https://doi.org/10.1016/j.onano.2022.100043.
Khan MM, Madni A, Filipczak N, Pan J, Rehman M, Rai N, Attia SA, Torchilin VP. Folate targeted lipid chitosan hybrid nanoparticles for enhanced anti-tumor efficacy. Nanomedicine: Nanotechnology, Biology and Medicine., 28, 102228 (2020) https://doi.org/10.1016/j.nano.2020.102228.
San HH, Alcantara KP, Bulatao BP, Sorasitthiyanukarn FN, Nalinratana N, Suksamrarn A, Vajragupta O, Rojsitthisak P, Rojsitthisak P. Folic acid-grafted chitosan-alginate nanocapsules as effective targeted nanocarriers for delivery of turmeric oil for breast cancer therapy. Pharmaceutics, 15, 110 (2022) https://doi.org/10.3390/pharmaceutics15010110.
Murthy A, Ravi PR, Kathuria H, Vats R. Self-assembled lecithin-chitosan nanoparticles improve the oral bioavailability and alter the pharmacokinetics of raloxifene. Int J Pharm., 588, 119731 (2020) https://doi.org/10.1016/j.ijpharm.2020.119731.
Shafique M, Ur Rehman M, Kamal Z, Alzhrani RM, Alshehri S, Alamri AH, Bakkari MA, Sabei FY, Safhi AY, Mohammed AM, Hamd MAE, Almawash S. Formulation development of lipid polymer hybrid nanoparticles of doxorubicin and its in-vitro, in-vivo and computational evaluation. Front Pharmacol., 14, 1025013 (2023) https://doi.org/10.3389/fphar.2023.1025013.
Yadav B, Chauhan M, Dinkar R, Shekhar S, Singh RP. In silico modeling, development, characterization, in-vitro cytotoxicity, pharmacokinetic, and toxicological studies of folate-receptor targeted micelles containing cisplatin and upconversion nanoparticles for lung cancer therapy. Materials Today Communications, 39, 109007 (2024) https://doi.org/10.1016/j.mtcomm.2024.109007.
Li J, Zhang Z, Huang X. Tripterine and all-trans retinoic acid (ATRA) - loaded lipid-polymer hybrid nanoparticles for synergistic anti-arthritic therapy against inflammatory arthritis. Artif Cells Nanomed Biotechnol., 49, 575-585 (2021) https://doi.org/10.1080/21691401.2021.1964983.
Aman RM, Zaghloul RA, Elsaed WM, Hashim IIA. In vitro-in vivo assessments of apocynin-hybrid nanoparticle-based gel as an effective nanophytomedicine for treatment of rheumatoid arthritis. Drug Deliv Transl Res., 13, 2903-2929 (2023) https://doi.org/10.1007/s13346-023-01360-5.
Chauhan M, Singh RP, Sonali, Yadav B, Shekhar S, Kumar L, Mehata AK, Jhawat V, Dutt R, Garg V, Kailashiya V, Muthu MS. Dual-targeted transferrin and AS1411 aptamer conjugated micelles for improved therapeutic efficacy and imaging of brain cancer. Colloids and Surf. B: Biointerfaces, 231, 113544 (2023) https://doi.org/10.1016/j.colsurfb.2023.113544.
Negi S, Tandel N, Garg NK, Sharma P, Kumar R, Sharma P, Kumar R, Saini S, Sharma A, Tyagi RK. Co-delivery of aceclofenac and methotrexate nanoparticles presents an effective treatment for rheumatoid arthritis. International Journal of Nanomedicine, 31, 2149-77 (2024) https://doi.org/10.2147/IJN.S439359.
Mushtaq RY, Naveen NR, Rolla KJ, Al Shmrany H, Alshehri S, Salawi A, Kurakula M, Alghamdi MA, Rizg WY, Bakhaidar RB, Abualsunun WA. Design and evaluation of magnetic-targeted bilosomal gel for rheumatoid arthritis: flurbiprofen delivery using superparamagnetic iron oxide nanoparticles. Frontiers in Pharmacology, 15, 1433734 (2024) https://doi.org/10.3389/fphar.2024.1433734.
Zhao J, Zhang X, Sun X, Zhao M, Yu C, Lee RJ, Sun F, Zhou Y, Li Y, Teng L. Dual-functional lipid polymeric hybrid pH-responsive nanoparticles decorated with cell penetrating peptide and folate for therapy against rheumatoid arthritis. Eur. J Pharm Biopharm., 130, 39-47 (2018) https://doi.org/10.1016/j.ejpb.2018.06.020.
Song Y, Yang P, Guo W, Lu P, Huang C, Cai Z, Jiang X, Yang G, Du Y, Zhao F. Supramolecular Hydrogel Dexamethasone–Diclofenac for the Treatment of Rheumatoid Arthritis. Nanomaterials., 14, 645 (2024) https://doi.org/10.3390/nano14070645.
Al-Nemrawi N, Altawabeyeh R, Darweesh RS, Alnabulsi S. Coating methotrexate-PLGA nanoparticles with folic acid-chitosan conjugate for cancer targeting. Pharmacia, 71, 1-9 (2024) https://doi.org/10.3897/pharmacia.71.e120072.
Singh R, Jadhav K, Kamboj R, Malhotra H, Ray E, Jhilta A, Dhir V, Verma RK. Self-actuating inflammation responsive hydrogel microsphere formulation for controlled drug release in rheumatoid arthritis (RA): Animal trials and study in human fibroblast like synoviocytes (hFLS) of RA patients. Biomaterials Advances., 160, 213853 (2024) https://doi.org/10.1016/j.bioadv.2024.213853.
Tahir N, Madni A, Balasubramanian V, Rehman M, Correia A, Kashif PM, Mäkilä E, Salonen J, Santos HA. Development and optimization of methotrexate-loaded lipid-polymer hybrid nanoparticles for controlled drug delivery applications. International journal of pharmaceutics, 533, 156-68 (2017) https://doi.org/10.1016/j.ijpharm.2017.09.061.
Bharti C, Nagaich U, Pandey J, Jain S, Jain N. Development of nitazoxanide-loaded colon-targeted formulation for intestinal parasitic infections: centre composite design-based optimization and characterization. Future Journal of Pharmaceutical Sciences, 6, 119 (2020) https://doi.org/10.1186/s43094-020-00130-1.
Zarei K, Jahanbakhshi M, Nahavandi R, Emadi R. Optimized co-delivery of curcumin and methylprednisolone using polyvinyl alcohol-coated CuO nanoparticles for synergistic rheumatoid arthritis treatment. Heliyon, 10 (2024) https://doi.org/10.1016/j.heliyon.2024.e40429.
Zamanian MY, Zafari H, Osminina MK, Skakodub AA, Al‐Aouadi RF, Golmohammadi M, Nikbakht N, Fatemi I. Improving dexamethasone drug loading and efficacy in treating rheumatoid arthritis via liposome: Focusing on inflammation and molecular mechanisms. Animal Models and Experimental Medicine, 8, 5-19 (2025) https://doi.org/10.1002/ame2.12518.
Bae Y, Zeb A, Choi HI, Ryu JS, Gul M, Noh HY, Cho J, Gil J, Shah FA, Chang SY, Bae ON. High payload dexamethasone palmitate-loaded solid lipid nanoparticles for enhanced anti-inflammatory effects in acute skin inflammation model. Journal of Pharmaceutical Investigation, 54, 617-29 (2024) https://doi.org/10.1007/s40005-024-00674-x.
Siddiqui B, ur Rehman A, Gul R, Chaudhery I, Shah KU, Ahmed N. Folate decorated chitosan-chondroitin sulfate nanoparticles loaded hydrogel for targeting macrophages against rheumatoid arthritis. Carbohydrate polymers, 327, 121683 (2024) https://doi.org/10.1016/j.carbpol.2023.121683.
Published
How to Cite
Issue
Section
Copyright (c) 2025 Ekta Panchal, Shiv Kumar Yadav, Neha Jain, Mahima Chauhan, Archana Sharma

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
















