Development and evaluation of non-saccharide polymer-based taste-masked clarithromycin tablets: a novel approach to improve pediatric compliance
DOI:
https://doi.org/10.69857/joapr.v13i5.1141Keywords:
Clarithromycin, Taste masking, Non-saccharide polymers, Pediatric compliance, Eudragit, Tablet formulationAbstract
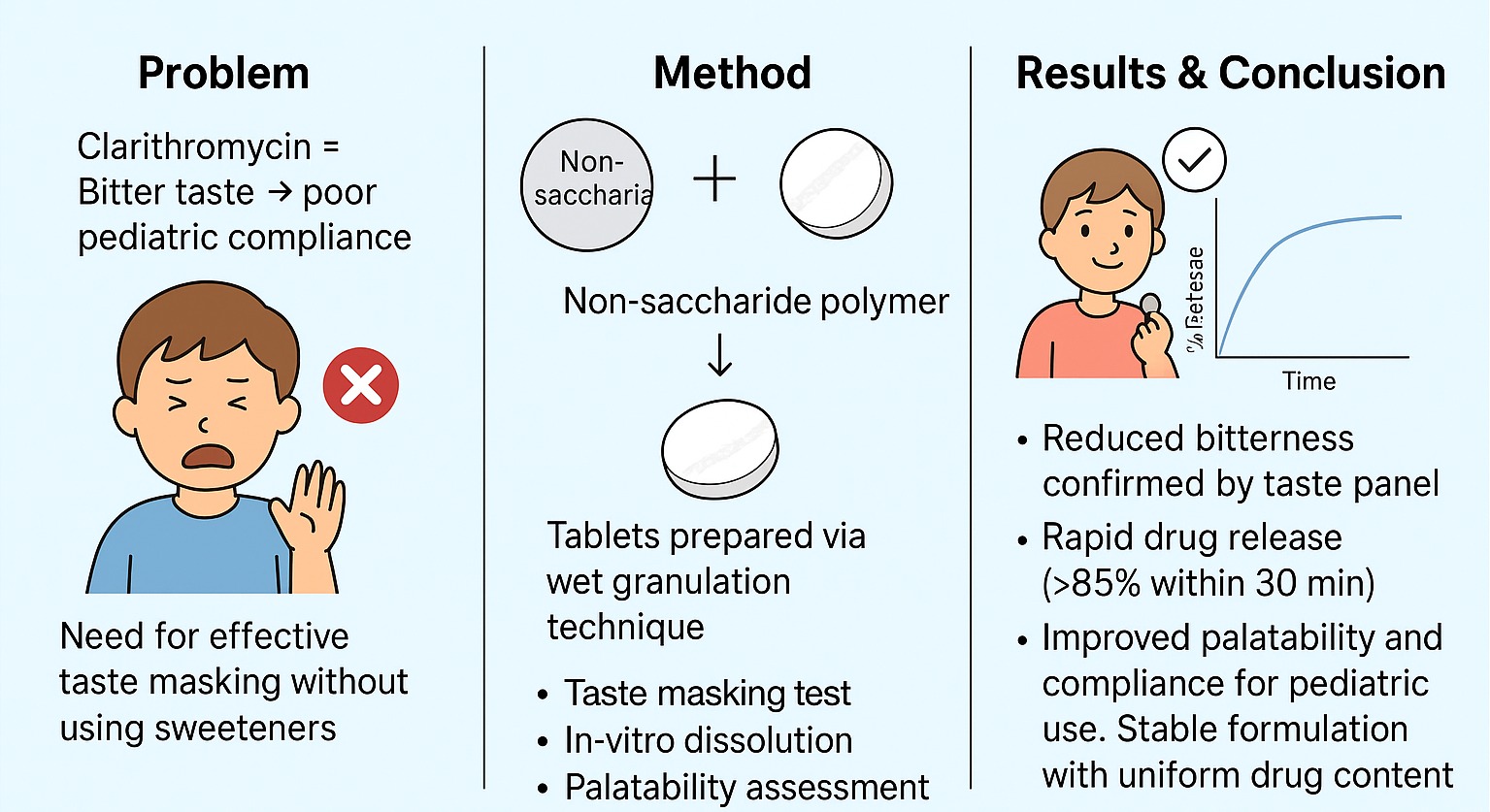
Background: Clarithromycin, a macrolide antibiotic, is commonly prescribed for respiratory and skin infections. However, its intensely bitter taste significantly hampers patient compliance, especially in pediatric and geriatric populations. The present study focused on formulating a novel taste-masked clarithromycin tablet using non-saccharide polymers to improve palatability while maintaining pharmacological performance. Methodology: Taste masking was accomplished by employing Eudragit E-100, Ethyl Cellulose, and Hydroxypropyl-β-Cyclodextrin (HP-β-CD) as coating agents. Granules were formulated using a bottom-spray fluidized bed coating process, followed by compression into tablets. These were assessed for physical parameters, disintegration time, in vitro drug release, and sensory evaluation using an electronic tongue system. The dissolution behavior was compared with that of a marketed clarithromycin formulation. Stability testing was conducted under accelerated (40°C/75% RH) and long-term (25°C/60% RH) storage conditions. Result and Discussion: The optimized formulation (Batch B9) demonstrated complete taste masking, exhibiting a bitterness score of 0 on the electronic tongue. Sensory data projected an over 85% improvement in patient acceptability. The tablets disintegrated within 60 seconds and showed a drug release of 98.1% within 30 minutes. Comparative dissolution profiling indicated no statistically significant difference (p > 0.05) between the test and reference products. Stability studies confirmed robust physicochemical stability for six months under both storage conditions. Conclusion: The study successfully developed a taste-masked clarithromycin tablet employing non-saccharide polymers, ensuring effective taste masking, rapid disintegration, and consistent drug release. This formulation offers a promising approach to enhance treatment compliance, particularly in populations sensitive to taste.
Downloads
References
Lohsen S, Stephens DS. Current macrolide antibiotics and their mechanisms of action. Antibiotic Drug Resist., 9, 97–117 (2019) https://doi.org/10.1002/9781119282549.ch5.
Krawczyk SJ, Leśniczak-Staszak M, Gowin E, Szaflarski W. Mechanistic insights into clinically relevant ribosome-targeting antibiotics. Biomolecules, 14(10), 1263 (2024) https://doi.org/10.3390/biom14101263.
Villa Zapata L, Hansten PD, Horn JR, Boyce RD, Gephart S, Subbian V, Romero A, Malone DC. Evidence of clinically meaningful drug–drug interaction with concomitant use of colchicine and clarithromycin. Drug Saf., 43, 661–68 (2020) https://doi.org/10.1007/s40264-020-00930-7.
Lester S, Kleijn M, Cornacchia L, Hewson L, Taylor MA, Fisk I. Factors affecting adherence, intake, and perceived palatability of oral nutritional supplements: a literature review. J. Nutr. Health Aging, 26(7), 663–74 (2022) https://doi.org/10.1007/s12603-022-1819-3.
Yir-Erong B, Bayor MT, Ayensu I, Gbedema SY, Boateng JS. Oral thin films as a remedy for noncompliance in pediatric and geriatric patients. Ther. Deliv., 10(7), 443–64 (2019) https://doi.org/10.4155/tde-2019-0032.
Soares N, Mitchell R, McGoff T, Bailey T, Wellman GS. Taste perceptions of common pediatric antibiotic suspensions and associated prescribing patterns in medical residents. J. Pediatr. Pharmacol. Ther., 27(4), 316–23 (2022) https://doi.org/10.5863/1551-6776-27.4.316.
Priyadarshan KR, Sahu A, Mahapatra AK, Chowdary KA, Nahak A, Patra RK. Rivaroxaban solid dispersions for dissolution enhancement and formulation of mouth disintegrating tablets. J. Appl. Pharm. Res., 12, 75–82 (2024) https://doi.org/10.69857/joapr.v12i4.647.
Ogbonna JD, Cunha E, Attama AA, Ofokansi KC, Ferreira H, Pinto S, Gomes J, Marx ÍM, Peres AM, Lobo JM, Almeida IF. Overcoming challenges in pediatric formulation with a patient-centric design approach: a proof-of-concept study on the design of an oral solution of a bitter drug. Pharmaceuticals, 15(11), 1331 (2022) https://doi.org/10.3390/ph15111331.
Kothawade SN, Pande VV. Formulation optimization of solid dispersion of candesartan cilexetil. Asian J. Pharm. Res. Health Care, 15(1), 59–63 (2023) https://doi.org/10.4103/ajprhc.ajprhc_104_22.
Wang N, Shi H, Yang S. 3D printed oral solid dosage form: modified release and improved solubility. J. Control. Release, 351, 407–31 (2022) https://doi.org/10.1016/j.jconrel.2022.09.023.
Turac IR, Porfire A, Iurian S, Crișan AG, Casian T, Iovanov R, Tomuță I. Expanding the manufacturing approaches for gastroretentive drug delivery systems with 3D printing technology. Pharmaceutics, 16(6), 790 (2024) https://doi.org/10.3390/pharmaceutics16060790.
Kothawade SN, Chaudhari PD. Development of biodegradable porous starch foam for improving oral delivery of eprosartan mesylate. J. Adv. Sci. Res., 12(03), 120–26 (2021) https://doi.org/10.55218/JASR.s1202112314.
Colombo G, Politis S, Rossi A. Technologies and mechanisms for oral modified release by monolithic and multiparticulate delivery systems. Oral Drug Deliv. Modif. Release Formul., 20(1), 119–36 (2022) https://doi.org/10.1002/9781119772729.ch7.
Lakumalla D, Podichety N, Maddali RK. Design and Characterization of Glimepiride Hydrotropic Solid Dispersion To Enhance the Solubility and Dissolution. J. Appl. Pharm. Res., 12, 68–78 (2024) https://doi.org/10.18231/j.joapr.2024.12.2.68.78.
Ghazi N, Liu Z, Bhatt C, Kiang S, Cuitino A. Investigating the effect of APAP crystals on tablet behavior manufactured by direct compression. AAPS PharmSciTech, 20, 1–4 (2019) https://doi.org/10.1208/s12249-019-1369-0.
Kadota K, Terada H, Fujimoto A, Nogami S, Uchiyama H, Tozuka Y. Formulation and evaluation of bitter taste-masked orally disintegrating tablets of high memantine hydrochloride loaded granules coated with polymer via layering technique. Int. J Pharm, 604, 120725 (2021) https://doi.org/10.1016/j.ijpharm.2021.120725.
Ranmal SR, Lavarde M, Wallon E, Issa S, Taylor WR, Nguyen Ngoc Pouplin JL, Tuleu C, Pensé-Lhéritier AM. Responsive sensory evaluation to develop flexible taste-masked paediatric primaquine tablets against malaria for low-resource settings. Pharmaceutics, 15(7), 1879 (2023) https://doi.org/10.3390/pharmaceutics15071879.
Chiarugi I, Biagi D, Nencioni P, Maestrelli F, Valleri M, Mura PA. Taste masking of dexketoprofen trometamol orally disintegrating granules by high-shear coating with glyceryl distearate. Pharmaceutics, 16(2), 165 (2024) https://doi.org/10.3390/pharmaceutics16020165.
Hu S, Liu X, Zhang S, Quan D. An overview of taste-masking technologies: approaches, application, and assessment methods. AAPS PharmSciTech, 24(2), 67 (2023)
https://doi.org/10.1208/s12249-023-02520-z.
Shirwar M, Birajdar S, Garad S, Kumbhar S. Development and validation of novel UV-visible spectrophotometric method for estimation of tepotinib in bulk and in pharmaceutical formulation. Int. J. Pharm. Pharm. Sci., 15(9), 32–36 (2023) https://dx.doi.org/10.22159/ijpps.2023v15i9.48431.
Lex TR, Rodriguez JD, Zhang L, Jiang W, Gao Z. Development of in vitro dissolution testing methods to simulate fed conditions for immediate release solid oral dosage forms. AAPS J., 24(2), 40 (2022) https://doi.org/10.1208/s12248-022-00690-5.
Boniatti J, Tappin MR, da S Teixeira RG, de AV Gandos T, Rios LP, Ferreira IA, Oliveira KC, Calil-Elias S, Santana AK, da Fonseca LB, Shimizu FM. In vivo and in vitro taste assessment of artesunate–mefloquine, praziquantel, and benznidazole drugs for neglected tropical diseases and pediatric patients. AAPS PharmSciTech, 23, 1–0 (2022) https://doi.org/10.1208/s12249-021-02162-z.
Guedes MD, Marques MS, Guedes PC, Contri RV, Kulkamp Guerreiro IC. The use of electronic tongue and sensory panel on taste evaluation of pediatric medicines: a systematic review. Pharm. Dev. Technol., 26(2), 119–37 (2021) https://doi.org/10.1080/10837450.2020.1860088.
Siafaka P, Ipekci E, Caglar EŞ, Okur NU, Buyukkayhan D. Current status of pediatric formulations for chronic and acute children’s diseases: applications and future perspectives. Medeni Med. J., 36(2), 152–62 (2021) https://doi.org/10.5222/MMJ.2021.78476.
Scrivens G. Prediction of the long-term dissolution performance of an immediate-release tablet using accelerated stability studies. J. Pharm. Sci., 108(1), 506–15 (2019) https://doi.org/10.1016/j.xphs.2018.10.025.
Published
How to Cite
Issue
Section
Copyright (c) 2025 Mahesh Bhalsing, Deshraj Chumbhale

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
















