Mechanical and dissolution properties of Eudragit L100 and S100 films in buffer solutions
DOI:
https://doi.org/10.69857/joapr.v13i4.1054Keywords:
MAA: MMA film, Mechanical propertie, Dissolution rate, Buffer capacity, Enteric coating, Concentration gradientsAbstract
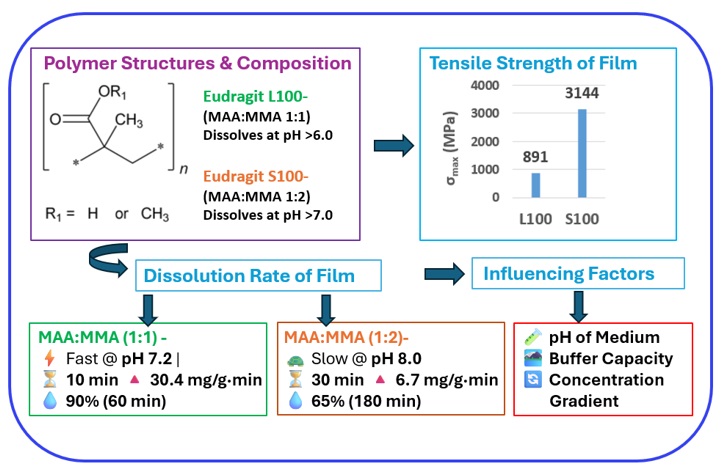
Background: Methacrylic acid (MAA) and methyl methacrylate (MMA) affect the mechanical and dissolution properties of enteric polymers, such as Eudragit L100 and S100. Their composition determines polymer flexibility, strength, and solubility, which are critical for pharmaceutical enteric coatings. This study examines the impact of the MAA: MMA ratio on the mechanical and dissolution properties of Eudragit L100 (1:1) and Eudragit S100 (1:2) films. Methodology: Mechanical testing assessed stiffness, tensile strength, and flexibility. Dissolution studies evaluated solubility at different pH levels, measuring peak dissolution rates. Results and Discussion: Eudragit L100, with more MAA, was stiffer and more brittle, while Eudragit S100 had higher tensile strength but reduced flexibility. Acidic conditions weakened both, due to water interactions with MAA. Eudragit L100 dissolved rapidly at pH 7.2 (90% mass loss in 60 min, peak 30.4 mg/g·min at 10 min), whereas Eudragit S100 showed minimal dissolution at lower pH, but dissolved significantly at pH 8.0 (64.5% at 180 min, peak 6.7 mg/g·min at 30 min). Larger dissolution volumes, maintained concentration gradients, enhancing dissolution, while high-capacity buffers stabilized pH and improved solubility. Conclusion: MAA: MMA composition critically affects the mechanical and dissolution properties of Eudragit L100 and S100, with concentration gradients playing a key role in dissolution, informing their application in enteric coatings.
Downloads
References
Saxena P, Shukla P. A comparative analysis of the basic properties and applications of poly (vinylidene fluoride) (PVDF) and poly (methyl methacrylate) (PMMA). Polymer Bulletin, 79, 5635–5665 (2022) https://doi.org/10.1007/s00289-021-03790-y
Sung YK, Kim SW. Recent advances in polymeric drug delivery systems. Biomater Res, 24, 12 (2020) https://doi.org/10.1186/s40824-020-00190-7
Mohosin K, Vahora NS. Investigating the Impact of Tablet Coatings on Gastrointestinal Tract Residence Time and Drug Bioavailability: A Comparative Study of Different Coating Materials. Journal of Angiotherapy, 8(12), 1–10 (2024) https://doi.org/10.25163/angiotherapy.81210103
Gvozdeva Y, Staynova R. pH-Dependent Drug Delivery Systems for Ulcerative Colitis Treatment. Pharmaceutics, 17(2), 226 (2025) https://doi.org/10.3390/pharmaceutics17020226
Nikam A, Sahoo PR, Musale S, Pagar RR, Paiva-Santos AC, Giram PS. A Systematic Overview of Eudragit® Based Copolymer for Smart Healthcare. Pharmaceutics, 15(2), 587 (2023) https://doi.org/10.3390/pharmaceutics15020587
Loh YY, Enose AA, Garg V. Enteric-Coated Polymers Past and Present - A Review. Drug Deliv Lett, 12(2), 85–95 (2022) https://doi.org/10.2174/2210303112666220413081911
Lu Y, Yamago S. Synthesis of Structurally Controlled, Highly Branched Polymethacrylates by Radical Polymerization through the Design of a Monomer Having Hierarchical Reactivity. Macromolecules, 53(8), 3209–3216 (2020) https://doi.org/10.1021/acs.macromol.0c00393
Van GL, Chourpa I, Gaigne C, Munnier E. Polymer-Based Smart Drug Delivery Systems for Skin Application and Demonstration of Stimuli-Responsiveness. Polymers (Basel), 13(8), 1285 (2021) https://doi.org/10.3390/polym13081285
Patra ChN, Priya R, Swain S, Kumar Jena G, Panigrahi KC, Ghose D. Pharmaceutical significance of Eudragit: A review. Futur J Pharm Sci, 3(1), 33–45 (2017) https://doi.org/10.1016/j.fjps.2017.02.001
Wang X, Wang M, Wang Q, Yuan Y, Hao Q, Bi Y, He Y, Zhao J, Hao J. Fabrication and in vitro/in vivo characterization of Eudragit enteric nanoparticles loaded with indomethacin. Chemical Papers, 76, 1119–1133 (2022) https://doi.org/10.1007/s11696-021-01921-3
dos Santos J, da Silva GS, Velho MC, Beck RCR. Eudragit®: A Versatile Family of Polymers for Hot Melt Extrusion and 3D Printing Processes in Pharmaceutics. Pharmaceutics, 13(9), 1424 (2021) https://doi.org/10.3390/pharmaceutics13091424
United States Pharmacopeia (2023). NF Monographs, Methacrylic Acid and Methyl Methacrylate Copolymer. USP-NF. Rockville, MD: United States Pharmacopeia. Doc ID: GUID-67410784-4D2C-417C-9854-60E8F091A1CC_4_en-US, cited 10 August 2023. Https://Doi.Org/10.31003/USPNF_M4367_04_01
Ibrahim M, Sarhan HA, Naguib YW, Abdelkader H. Design, characterization and in vivo evaluation of modified release baclofen floating coated beads. Int J Pharm, 582, 119344 (2020) https://doi.org/10.1016/j.ijpharm.2020.119344
United States Pharmacopeia (2023). USP Monographs, Mesalamine Delayed-Release Tablets. USP-NF. Rockville, MD: United States Pharmacopeia. Doc ID: GUID-3DD61839-B554-4868-AA34-7326951B1B63_8_en-US, cited 10 July 2023. Https://Doi.Org/10.31003/USPNF_M49460_08_01
Shohag MH, Kuddus SA, Brishty EMS, Chowdhury SS, Hossain MT, Hasan M, Khan SI, Hossain M, Reza HM. Post-market quality assessment of 22 ciprofloxacin brands by HPLC available in Bangladesh market. Heliyon, 9, e17180 (2023) https://doi.org/10.1016/j.heliyon.2023.e17180
United States Pharmacopeia (2023). General Chapter, 〈711〉 Dissolution. USP-NF. Rockville, MD: United States Pharmacopeia. Doc ID: GUID-AC788D41-90A2-4F36-A6E7-769954A9ED09_3_en-US, cited 10 August 2023. Https://Doi.Org/10.31003/USPNF_M99470_03_01
United States Pharmacopeia (2024). USP Monographs NF Monographs, Purified Water. USP-NF. Rockville, MD: United States Pharmacopeia. Doc ID: GUID-32745524-1141-4BE6-A3B6-A96B3368461D_4_en-US, cited 15 February 2024. Https://Doi.Org/10.31003/USPNF_M88890_04_01
United States Pharmacopeia (2024). Reagents, 0.1 N Hydrochloric Acid VS. USP-NF. Rockville, MD: United States Pharmacopeia. Doc ID: GUID-C7B9ED83-E765-442F-9011-74C890C01AD8_2_en-US, cited 12 February 2024. Https://Doi.Org/10.31003/USPNF_R5972_02_01
United States Pharmacopeia (2024). Reagents, Buffer Solutions. USP-NF. Rockville, MD: United States Pharmacopeia. Doc ID: GUID-0E4CE941-0762-456C-94B0-9209A58834FC_3_en-US, cited 12 February 2024. Https://Doi.Org/10.31003/USPNF_R2999_03_01
Singh KK, Raghuvanshi RS, Asif M. The exploring role and responsibility of Indian pharmacopoeia commission: An introduction. Journal of Education Technology in Health Sciences, 10, 64–8 (2024) https://doi.org/10.18231/j.jeths.2023.015
Henman MC, Ravera S, Lery F-X. Council of Europe Resolution on the Implementation of Pharmaceutical Care—A Step Forward in Enhancing the Appropriate Use of Medicines and Patient-Centred Care. Healthcare, 12, 232 (2024) https://doi.org/10.3390/healthcare12020232
Lucy CA. Is Your Henderson–Hasselbalch Calculation of Buffer pH Correct? J Chem Educ, 100, 2418–2422 (2023) https://doi.org/10.1021/acs.jchemed.2c01203
United States Pharmacopeia (2024). Reagents, Buffer Solutions. USP-NF. Rockville, MD: United States Pharmacopeia. Doc ID: GUID-0E4CE941-0762-456C-94B0-9209A58834FC_3_en-US. https://doi.org/10.31003/USPNF_R2999_03_01
Eslami Z, Elkoun S, Robert M, Adjallé K. A Review of the Effect of Plasticizers on the Physical and Mechanical Properties of Alginate-Based Films. Molecules, 28(18), 6637 (2023) https://doi.org/10.3390/molecules28186637
Published
How to Cite
Issue
Section
Copyright (c) 2025 Rashmi Chauhan, Vaibhav Jindas Ambudkar

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
















