Formulation and evaluation of moxifloxacin-loaded proniosomal gel for ocular delivery
DOI:
https://doi.org/10.69857/joapr.v13i5.1052Keywords:
Ocular drug delivery, Moxifloxacin HCl, Bioavailabilit, Proniosomal GelAbstract
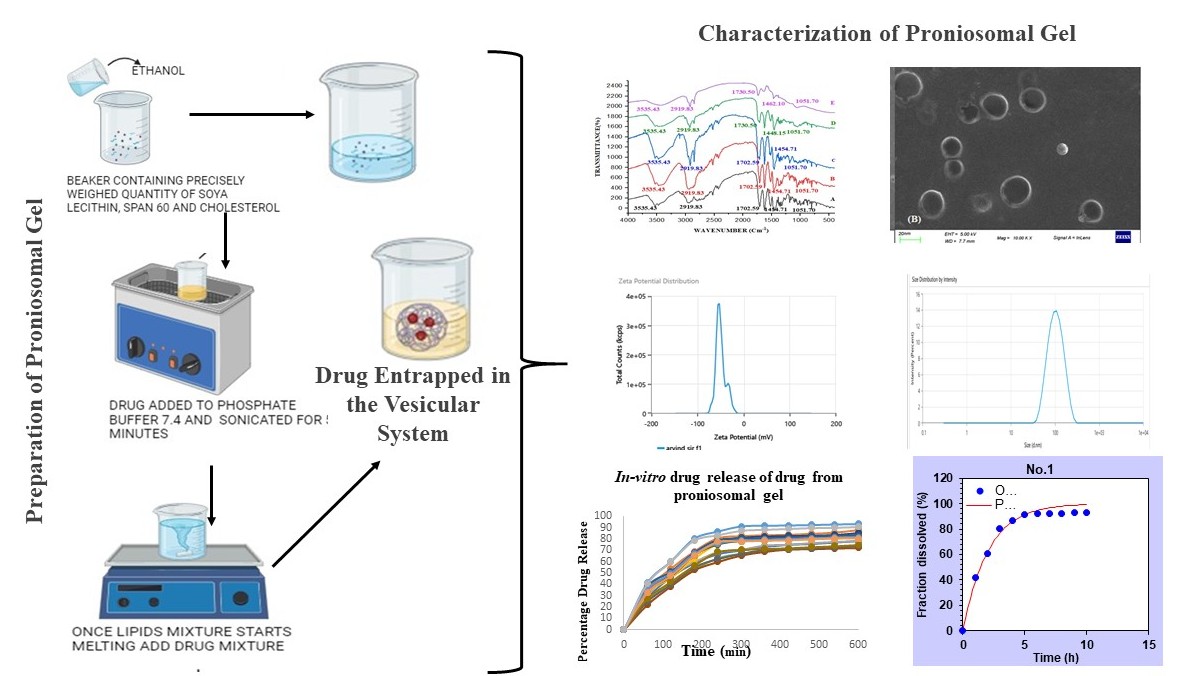
Background: The management of ocular disorders is particularly arduous due to the eye's distinctive anatomy. The cornea serves as a crucial obstacle to medication absorption, restricting the effectiveness of conventional dosage regimens. To address this issue, a proniosomal gel has been developed, comprising a lipid bilayer that emulates the corneal cell membrane, thereby enhancing drug transport across the cornea and resulting in improved bioavailability. Methodology: The Moxifloxacin-loaded Proniosomal gel was developed by the coacervation phase separation method. To determine the physicochemical characteristics of the gel, various evaluation parameters were conducted, including viscosity, pH, FTIR, zeta potential, polydispersity index (PDI), particle size (PS), entrapment efficacy (EE), SEM, and in vitro studies. Results and Discussion: The F5 optimized formulation exhibited a maximum EE of 94.47±0.23%, an ideal pH of 6.8, a PS of 105.4 nm, a PDI of 0.3678, and a zeta potential within ±30 mV. In-vitro drug release and kinetic studies showed that proniosomal gel followed first-order kinetic characteristics of drug released and a biphasic drug release pattern (There is an initial rapid release of the drug, followed by a slower, controlled release over an extended period). Conclusion: Proniosomal gels as drug delivery carriers increased corneal contact, penetration, and retention time in the eye, resulting in sustained action and increased bioavailability.
Downloads
References
Robert P, Robert PY, Rocher M. Ocular anatomy and physiology. Actualites Pharmaceutiques, 61 (620),16-20 (2022) https://doi.org/10.1016/j.actpha.2022.09.009.
Kumar S, Jatav DrRK, Shukla DrK. Fabrication and evaluation of Oxymetazoline Hydrochloride Proniosomal Gel as a Vesicular Drug Delivery System. Journal for ReAttach Therapy and Developmental Diversities, 6(1), 1079–1084 (2023) https://doi.org/10.53555/jrtdd.v6i1.2626.
Batur E, Özdemir S, Durgun ME, Özsoy Y. Vesicular Drug Delivery Systems: Promising Approaches in Ocular Drug Delivery. Pharmaceuticals, 17(4), 511 (2024) https://doi.org/10.3390/PH17040511.
Velchuri S, P RB, G SK, Sarvepalli R. Formulation and evaluation of moxifloxacin loaded ocular In-situ gels. Future Journal of Pharmaceuticals and Health Sciences, 4(1), 51-60 (2024) https://doi.org/10.26452/fjphs.v4i1.559.
Ahmed S, Farag MM, Attia H, Balkhi B, Adel IM, Nemr AA. Exploring the potential of antifungal-loaded proniosomes to consolidate corneal permeation in fungal keratitis: A comprehensive investigation from laboratory characterization to microbiological evaluation. Int J Pharm X, 9, 100322 (2025) https://doi.org/10.1016/J.IJPX.2025.100322.
Youssef AAA, Cai C, Dudhipala N, Majumdar S. Design of Topical Ocular Ciprofloxacin Nanoemulsion for the Management of Bacterial Keratitis. Pharmaceuticals,14(3), 210 (2021) https://doi.org/10.3390/PH14030210.
Kandpal N, Ale Y, Semwal YC, Padiyar N, Jakhmola V, Farswan AS, Nainwal N. Proniosomes: A provesicular system in ocular drug delivery. Journal of Advanced Biotechnology and Experimental Therapeutics, 6(3), 622–37 (2023) https://doi.org/10.5455/JABET.2023.D154.
Sharapova A, Ol’khovich M, Blokhina S, Zhirova E, Perlovich G. Solubility and partition behavior of moxifloxacin: Experimental results and thermodynamics properties. J Mol Liq, 339(5), 116814 (2021) https://doi.org/10.1016/j.molliq.2021.116814.
Tkachenko Y, Niedzielski P. FTIR as a Method for Qualitative Assessment of Solid Samples in Geochemical Research: A Review, Molecules,27(24), 8846 (2022) https://doi.org/10.3390/molecules27248846.
Rohman A, Ghazali MAB, Windarsih A, Irnawati, Riyanto S, Yusof FM, Mustafa S. Comprehensive Review on Application of FTIR Spectroscopy Coupled with Chemometrics for Authentication Analysis of Fats and Oils in the Food Products. Molecules, 25(22), 5485 (2020) https://doi.org/10.3390/molecules25225485.
Mohamed SA, Rofaeil RR, Salem H, Elrehany M, Asiri YI, Al Fatease A, Abdelkader H. Proniosomal Gel-Loaded Phosphodiesterase Inhibitors (Sildenafil, Vardenafil, and Tadalafil): Prospects for Topical Penile Therapy of Tadalafil for Treatment of Erectile Dysfunction. Gels, 9(8), 597 (2023) https://doi.org/10.3390/gels9080597.
Darson J, Thirunellai Seshadri R, Katariya K, Mohan M, Srinivas Kamath M, Etyala MA, Chandrasekaran G. Design development and optimisation of multifunctional Doxorubicin-loaded Indocynanine Green proniosomal gel derived niosomes for tumour management. Sci Rep, 13(1),1697 (2023) https://doi.org/10.1038/s41598-023-28891-8.
Silva D, de Sousa HC, Helena Gil M, Alvarez-Lorenzo C, Concheiro A, Saramago B, Paula Serro A. Moxifloxacin imprinted silicon based hydrogels for sustained ocular release. Ann Med, 51, (2024) https://doi.org/10.1080/07853890.2018.1562708.
Aboali FA, Habib DA, Elbedaiwy HM, Farid RM. Curcumin-loaded proniosomal gel as a biofreindly alternative for treatment of ocular inflammation: In-vitro and in-vivo assessment. Int J Pharm, 589(3), 119835 (2020) https://doi.org/10.1016/J.IJPHARM.2020.119835.
Bhageerathy A, Prasanth V V. Formulation and Evaluation of Moxifloxacin Hydrochloride Loaded Cubosomal Gel for Ocular Delivery. Journal of Drug Delivery and Therapeutics, 14(3), 88-99 (2024) https://doi.org/10.22270/jddt.v14i3.6464.
Vashist S, Gadewar MM. Preparation and Characterization of Mucoadhesive Proniosomal Gel of Curcumin with Thiolated Chitosan for the Treatment of Oral Mucositis. Int J Pharm Investig, 13(3), 666-672 (2023) https://doi.org/10.5530/ijpi.13.3.083.
Sai Sreenidhi K, Anand Kumar Yegnoor. Proniosomal gel mediated transdermal delivery of Donepezil HCl: Development and in vitro characterization. International Journal of Frontline Research in Pharma and Bio Sciences, 2(2), 17-23 (2023) https://doi.org/10.56355/ijfrpbs.2023.2.2.0018.
Gudalwar BR, Shukla TP. Design and Characterization of Calcipotriol Proniosomal Gel: In-vivo Exploration against Imiquimod-induced Psoriasis in Experimental Animals. International Journal of Drug Delivery Technology, 13(4), 1336-1341 (2023) https://doi.org/10.25258/ijddt.13.4.34.
Gentili V, Strazzabosco G, Spena R, Rizzo S, Beltrami S, Schiuma G, Alogna A, Rizzo R. Comparison Between Moxifloxacin and Chloramphenicol for the Treatment of Bacterial Eye Infections. Curr Ther Res Clin Exp, 100, 100740 (2024) https://doi.org/10.1016/j.curtheres.2024.100740.
Ayoub RK, Murtaza G, Imran M, Khan SA, Mir S, Khan AK, Azhar S, Mehmood Z, Sajjad A, Shah SNH. Formulation and permeation kinetic studies of flurbiprofen gel. Tropical Journal of Pharmaceutical Research, 14(142), 195 (2015) https://doi.org/10.4314/tjpr.v14i2.2.
Špaglová M, Papadakos M, Čuchorová M, Matušová D. Release of Tretinoin Solubilized in Microemulsion from Carbopol and Xanthan Gel: In Vitro versus Ex Vivo Permeation Study. Polymers (Basel), 15(2), 329 (2023) https://doi.org/10.3390/polym15020329.
Li S, Chen L, Fu Y. Nanotechnology-based ocular drug delivery systems: recent advances and future prospects, J Nanobiotechnol, 21, 232 (2023) https://doi.org/10.1186/s12951-023-01992-2
Duman G, Yıldır İ, Macit M, Genç E, Sümer E, Kale S, Deniz İ. Development and evaluation of 3D-printed ocular insert containing liposomal moxifloxacin. J Drug Deliv Sci Technol, 92, (2024) https://doi.org/10.1016/j.jddst.2024.105353.
Gudalwar BR, Shukla TP. Formulation Development and Characterization of Acitretin Proniosomal gel: In-vivo Exploration against Imiquimod-induced Psoriasis in Experimental Animals. International Journal of Pharmaceutical Quality Assurance, 14(4), 1011-1016 (2023) https://doi.org/10.25258/ijpqa.14.4.29.
Fahmy AM, El-Setouhy DA, Ibrahim AB, Habib BA, Tayel SA, Bayoumi NA. Penetration enhancer-containing spanlastics (PECSs) for transdermal delivery of haloperidol: in vitro characterization, ex vivo permeation and in vivo biodistribution studies. Drug Deliv, 25(1), 12–22 (2018) https://doi.org/10.1080/10717544.2017.1410262.
Attia HH, Shaker DS, ElMeshad A, El-Kayal M. Optimization and in-vitro assessment of the effectiveness of carvedilol-loaded proniosomal gels as a promising therapeutic approach for the topical treatment of skin cancer. J Drug Deliv Sci Technol, 86(5),104665 (2023) https://doi.org/10.1016/j.jddst.2023.104665.
Khalil RM, Abdelbary GA, Basha M, Awad GEA, El-Hashemy HA. Design and evaluation of proniosomes as a carrier for ocular delivery of lomefloxacin HCl. J Liposome Res, 27, 118–29 (2017) https://doi.org/10.3109/08982104.2016.1167737.
Spoorthy Narayandas, Anand Kumar Yegnoor. Formulation and evaluation of Oxybutynin chloride loaded proniosomal gel for transdermal drug delivery. Magna Scientia Advanced Research and Reviews, 9(1), 082-092 (2023) https://doi.org/10.30574/msarr.2023.9.1.0126.
Emad Eldeeb A, Salah S, Ghorab M. Proniosomal gel-derived niosomes: an approach to sustain and improve the ocular delivery of brimonidine tartrate; formulation, in-vitro characterization, and in-vivo pharmacodynamic study. Drug Deliv, 26(1), 509–21 (2019) https://doi.org/10.1080/10717544.2019.1609622.
Maanvizhi S, Iyyappan V, Bhavishi PG. In-vitro release study of diclofenac sodium from topical gel formulations using diffusion cell. Res J Pharm Technol, 13(6), 2901-2905 (2020) https://doi.org/10.5958/0974-360X.2020.00517.X.
El-Emam GA, Girgis GNS, El Sokkary MMA, El-Azeem Soliman OA, Abd El Gawad AEGH. Ocular inserts of voriconazole-loaded proniosomal gels: Formulation, evaluation and microbiological studies. Int J Nanomedicine, 15, 7825–40 (2020) https://doi.org/10.2147/IJN.S268208.
Abdelbary GA, Amin MM, Zakaria MY. Ocular ketoconazole-loaded proniosomal gels: formulation, ex vivo corneal permeation and in vivo studies. Drug Deliv, 24(1), 309-319 (2017) https://doi.org/10.1080/10717544.2016.1247928.
Published
How to Cite
Issue
Section
Copyright (c) 2025 Neha Kandpal, Yogita Ale, Kumari Kajal, Saurav Chamoli, Mansi Butola

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
















