Transdermal delivery of risedronate using chemical enhancers for improved skin penetration
DOI:
https://doi.org/10.69857/joapr.v13i4.1013Keywords:
Risedronate, Bisphosphonate, Enhancers, Transdermal deliveryAbstract
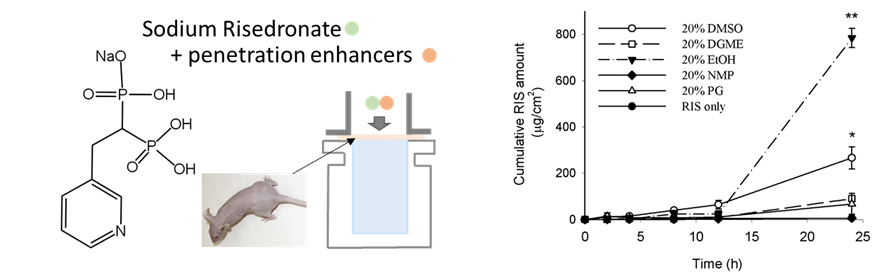
Background: Risedronate sodium (RIS) is effective for bone diseases but has low bioavailability and severe side effects. This study investigates the use of hydrophilic enhancers to improve the efficiency of RIS's transdermal delivery. Methods: This study involved preparing topical samples of RIS with various enhancers, including ethanol (EtOH), dimethyl sulfoxide (DMSO), Dimethylene glycol monomethyl ether (DGME), and propylene glycol (PG). In vitro permeation tests were conducted using hairless mouse skin in Franz diffusion cells, and skin irritation tests were performed on mice. Results: The cumulative amount of RIS after 24 hours significantly increased with penetration enhancers: 6.02 μg/cm² (RIS alone), 90.22 μg/cm² (20% DGME), 67.31 μg/cm² (20% PG), 266.31 μg/cm² (20% DMSO), and 784.52 μg/cm² (20% EtOH). EtOH showed a dose-dependent increase, with 1,302.76 μg/cm² at 50% concentration. Further experiments using DMSO and EtOH at concentrations of 5% and 10% identified the optimal permeation enhancement as follows: 201.36 ± 31.6 μg/cm2 (5% DMSO), 183.03 ± 31.6 μg/cm2 (10% DMSO), 261.71 ± 164.93 μg/cm2 (5% EtOH), 569.21 ± 197.67 μg/cm2 (10% EtOH). Discussion: EtOH and DMSO significantly enhanced RIS penetration by modifying the skin's structure. The study suggests that adjusting the concentration of these enhancers can control the penetration profile, offering a promising alternative to oral delivery. Conclusions: This study demonstrated that chemical enhancers significantly improved the skin penetration of RIS. The transdermal delivery of RIS can help reduce the side effects of oral delivery of the drug and thus improve patients’ compliance.
Downloads
References
Langdahl BL. Overview of treatment approaches to osteoporosis. Br. J. Pharmacol., 178, 1891-1906 (2021) https://doi.org/10.1111/bph.15024
Natesan V, Kim SJ. Metabolic bone diseases and new drug developments. Biomol Ther., 30, 309 (2022) https://doi.org/10.4062/biomolther.2022.007
Anish RJ, Nair A. Osteoporosis management-current and future perspectives–a systemic review. J. Orthop., 53, 101-113 (2024) https://doi.org/10.1016/j.jor.2024.03.002
Lu L, Lu L, Zhang J, Li J. Potential risks of rare serious adverse effects related to long‐term use of bisphosphonates: An overview of systematic reviews. J Clin Pharm Ther., 45, 45-51 (2020) https://doi.org/10.1111/jcpt.13056
Anastasilakis AD, et al. Osteonecrosis of the jaw and antiresorptive agents in benign and malignant diseases: a critical review organized by the ECTS. JCEM., 107, 1441-1460 (2022) https://doi.org/10.1210/clinem/dgab888
Chien HI, et al. Bisphosphonate-related osteonecrosis of the jaw. Ann. Plast. Surg., 86, S78-S83 (2021) https://doi.org/10.1097/SAP.0000000000002650
Dioguardi M, et al. Oral bisphosphonate-induced osteonecrosis complications in patients undergoing tooth extraction: a systematic review and literature updates. Eur Rev Med Pharmacol Sci., 27, 6359-6373 (2023) https://doi.org/10.26355/eurrev_202307_32996
Jeong WY, Kwon M, Choi HE, Kim KS. Recent advances in transdermal drug delivery systems: A review. Biomater. Res., 25, 1-15 (2021) https://doi.org/10.1186/s40824-021-00226-6
Yang, Y., et al. Recent advances in oral and transdermal protein delivery systems. Angew. Chem., 135, e202214795 (2023) https://doi.org/10.1002/ange.202214795
Karve T, et al. Long-acting transdermal drug delivery formulations: Current developments and innovative pharmaceutical approaches. Adv. Drug Deliv. Rev., 115326 (2024) https://doi.org/10.1016/j.addr.2024.115326
Sguizzato M, Esposito E, Cortesi R. Lipid-based nanosystems as a tool to overcome skin barrier. Int. J. Mol. Sci., 22, 8319 (2021) https://doi.org/10.3390/ijms22158319
Yu YQ, Yang X, Wu XF, Fan YB. Enhancing permeation of drug molecules across the skin via delivery in nanocarriers: novel strategies for effective transdermal applications. Front. Bioeng. Biotechnol., 9, 646554 (2021) https://doi.org/10.3389/fbioe.2021.646554
Zielińska M, et al. Determination of bisphosphonate properties in terms of bioavailability, bone affinity, and cytotoxicity. Pharmacol. Rep., 76, 1160–1173 (2024) https://doi.org/10.1007/s43440-024-00624-2.
Wang C, et al. Mitigating gastrointestinal side effects of risedronate sodium: A study on Bletilla striata polysaccharide microneedle patches. Int. J. Pharm., 25, 125609 (2025) https://doi.org/10.1016/j.ijpharm.2025.125609.
Gyanewali S, et al. Formulation development and in vitro–in vivo assessment of protransfersomal gel of anti-resorptive drug in osteoporosis treatment. Int. J. Pharm., 608, 121060 (2021) https://doi.org/10.1016/j.ijpharm.2021.121060.
Hmingthansanga V, et al. Improved topical drug delivery: Role of permeation enhancers and advanced approaches. Pharmaceutics, 14, 2818 (2022) https://doi.org/10.3390/pharmaceutics14122818.
Schafer N, Balwierz R, Biernat P, Ochędzan-Siodłak W, Lipok J. Natural ingredients of transdermal drug delivery systems as permeation enhancers of active substances through the stratum corneum. Mol. Pharm., 20, 3278–3297 (2023) https://doi.org/10.1021/acs.molpharmaceut.3c00126.
Sugumar V, Hayyan M, Madhavan P, Wong WF, Looi CY. Current development of chemical penetration enhancers for transdermal insulin delivery. Biomedicines, 11, 664 (2023) https://doi.org/10.3390/biomedicines11030664.
Anantrao JH, Nath PA, Nivrutti PR. Drug penetration enhancement techniques in transdermal drug delivery system: A review. J. Pharm. Res. Int., 33, 46–61 (2021) https://doi.org/10.9734/jpri/2021/v33i19B31337.
Vasyuchenko EP, Orekhov PS, Armeev GA, Bozdaganyan ME. CPE-DB: An open database of chemical penetration enhancers. Pharmaceutics, 13, 66 (2021) https://doi.org/10.3390/pharmaceutics13010066.
Hashemzadeh N, Jouyban A. Review of Pharmaceutical Applications of Diethylene Glycol-Monoethyl Ether. J. Pharm. Pharm. Sci., 25, 349–353 (2022) https://doi.org/10.18433/jpps32921.
Talole S, Godge R, Tambe N, Mhase N. Formulation and optimization of upadacitinib-loaded transdermal patches for rheumatoid arthritis with zero-order release kinetics. J. Appl. Pharm. Res., 13, 181–93 (2025) https://doi.org/10.69857/joapr.v13i2.1037.
Md Moshikur R, Goto M. Pharmaceutical Applications of Ionic Liquids: A Personal Account. TCR., 23, e202300026 (2023) https://doi.org/10.1002/tcr.202300026.
Mistry J, Notman R. Mechanisms of the drug penetration enhancer propylene glycol interacting with skin lipid membranes. J. Phys. Chem. B., 128, 3885–3897 (2024) https://doi.org/10.1021/acs.jpcb.3c06784.
Balmanno A, Falconer JR, Ravuri HG, Mills PC. Strategies to Improve the Transdermal Delivery of Poorly Water-Soluble Non-Steroidal Anti-Inflammatory Drugs. Pharmaceutics, 16, 675 (2024) https://doi.org/10.3390/pharmaceutics16050675.
Virani A, Puri V, Mohd H, Michniak-Kohn B. Effect of penetration enhancers on transdermal delivery of oxcarbazepine, an antiepileptic drug using microemulsions. Pharmaceutics, 15, 183 (2023) https://doi.org/10.3390/pharmaceutics15010183.
Karim M, Boikess RS, Schwartz RA, Cohen PJ. Dimethyl sulfoxide (DMSO): a solvent that may solve selected cutaneous clinical challenges. Arch. Dermatol. Res., 315, 1465–1472 (2023) https://doi.org/10.1007/s00403-022-02494-1.
Shinoda S, Tanigawa M, Sakuragi M. Permeation dynamics of microemulsions according to the amount of deep eutectic solvent when applied to the stratum corneum. RSC Adv., 15, 8977–8985 (2025) https://doi.org/10.1039/d5ra00403a.
Phatale V, et al. Overcoming skin barriers through advanced transdermal drug delivery approaches. JCR., 351, 361–380 (2022) https://doi.org/10.1016/j.jconrel.2022.09.025.
Fukuda K, et al. Three stepwise pH progressions in stratum corneum for homeostatic maintenance of the skin. Nat. Commun., 15, 4062 (2024) https://doi.org/10.1038/s41467-024-48226-z.
Boix-Montañés A, Celma-Lezcano C, Obach-Vidal R, Peraire-Guitart C. Collaborative permeation of drug and excipients in transdermal formulations. In vitro scrutiny for ethanol: limonene combinations. Eur J. Pharm. Biopharm., 181, 239–248 (2022) https://doi.org/10.1016/j.ejpb.2022.11.004.
Pires PC, Rodrigues M, Alves G, Santos AO. Strategies to improve drug strength in nasal preparations for brain delivery of low aqueous solubility drugs. Pharmaceutics, 14, 588 (2022) https://doi.org/10.3390/pharmaceutics14030588.
Iliopoulos F, Sil BC, Evans CL. The role of excipients in promoting topical and transdermal delivery: Current limitations and future perspectives. Front. Drug Deliv., 2, 1049848 (2022) https://doi.org/10.3389/fddev.2022.1049848
Chatterjee B, Reddy A, Santra M, Khamanga S. Amorphization of drugs for transdermal delivery-a recent update. Pharmaceutics, 14, 983 (2022) https://doi.org/10.3390/pharmaceutics14050983
Hao J, Chen C, Pavelic K, Ozer F. ZIF-8 as a pH-Responsive Nanoplatform for 5-Fluorouracil Delivery in the Chemotherapy of Oral Squamous Cell Carcinoma. Int. J. Mol. Sci., 25, 9292 (2024) https://doi.org/10.3390/ijms25179292
Published
How to Cite
Issue
Section
Copyright (c) 2025 So Hee Nam

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
















